Rare Isolate of Stephanoascus ciferrii from the Aural Discharge of Post-mastoidectomy Patient-A Case Report
Packia Nancy Romald1, Kopula Sathyamurthy Sridharan2, Sanjeev Mohanty3, Anupma Jyoti Kindo4
1 Postgraduate, Department of Microbiology, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India.
2 Professor, Department of Microbiology, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India.
3 Professor and Head, Department of Otorhinolaryngology, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India.
4 Professor and Head, Department of Microbiology, Sri Ramachandra Institute of Higher Education and Research, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Kopula Sathyamurthy Sridharan, Sri Ramachandra Medical College, Porur, Chennai-600116, Tamil Nadu, India.
E-mail: sridharshyama@gmail.com
Stephanoascus ciferrii is an ascomycetous yeast-like fungus, rarely causing human infections. It is also a teleomorph of Candida ciferrii. This species has a strong tendency to become resistant especially in patients on fluconazole prophylaxis. We report a case of post-mastoidectomy with continuous aural discharge not responding to topical and systemic treatment. Further characterisation showed ascospore formation and fluconazole resistance. Therefore, in vitro susceptibility testing is mandatory for the selection of appropriate antifungal drugs. This case report will alert the clinicians to look for fluconazole resistant yeast causing highly resistant aural infections.
Ascospores, Fluconazole resistant, Trichomonascus ciferrii
Case Report
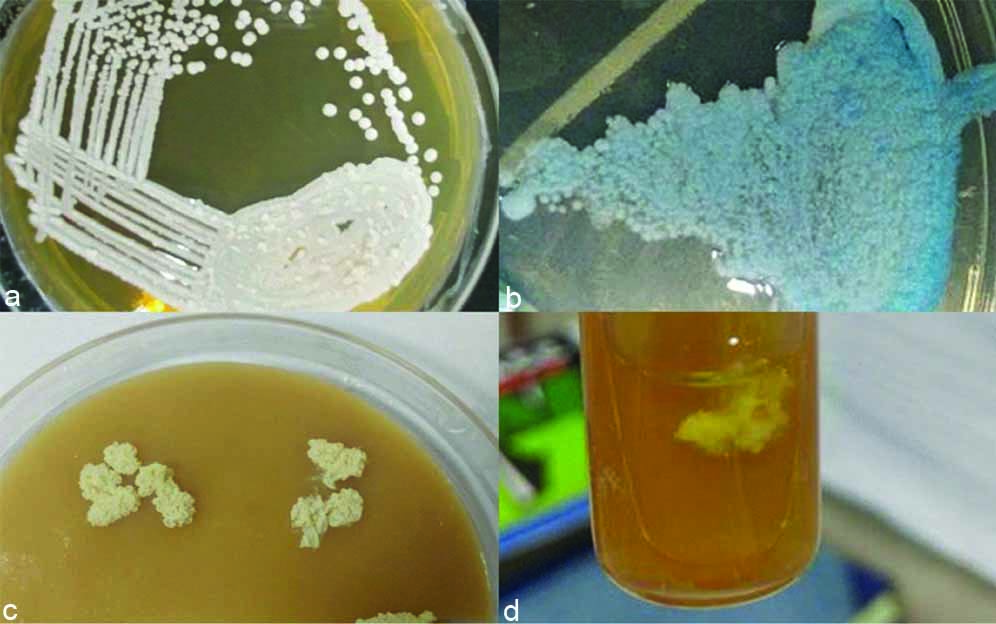
A 57-year-old female reported to the ENT OPD with complaints of excruciating ear pain, profuse ear discharge and hard of hearing in left ear for past three months. She had undergone left ear mastoidectomy for similar complaints 20 years back. She was apparently normal till three months back when she suddenly developed the above signs and symptoms. Patient did not have any significant past history of chronic disease, malignancy or other immunosuppressive status. Examination of the left ear showed healthy post aural scar, mastoid cavity was visualised and foul smelling thick discharge seen. Right ear was apparently normal. Ear swab was taken and sent for culture. Patient was admitted in hospital and was started on treatment with IV antibiotic. Laboratory parameters were within normal limits. Gram’s stain of ear swab showed few pus cells and budding yeast cell. Culture on Sabouraud’s Dextrose Agar (SDA) at 37°C showed white, rough, raised, wrinkled colonies, Hi-chrome agar showed dry blue-white, wrinkled colonies [Table/Fig-1]. Microscopic examination of the culture showed extensive branches and oval blastoconidia in chains of different sizes, arranged along pseudo-hyphae and true hyphae [Table/Fig-2]. Anti-fungal susceptibility by Kirby Bauer’s Disc diffusion method in accordance with CLSI guidelines M44-A2 (2009) was done for Fluconazole, Voriconazole, Itraconazole and Amphotericin-B. It showed resistance to Fluconazole and Amphotericin-B. Vitek 2 automated system ID reported Stephanoascus ciferrii resistant to Fluconazole. The patient was started on oral Voriconazole and topical Clotrimazole. Patient responded well and was discharged with same advice. The patient was followed up and did not have any similar complaints.
a) SDA showing white rough raised wrinkle colonies; b) Hi-chrome agar-showing dry blue-white, wrinkled colonies; c) Oat meal agar-showing rough, dry white, wrinkled colonies; d) Growth on SDA with olive oil overlay.
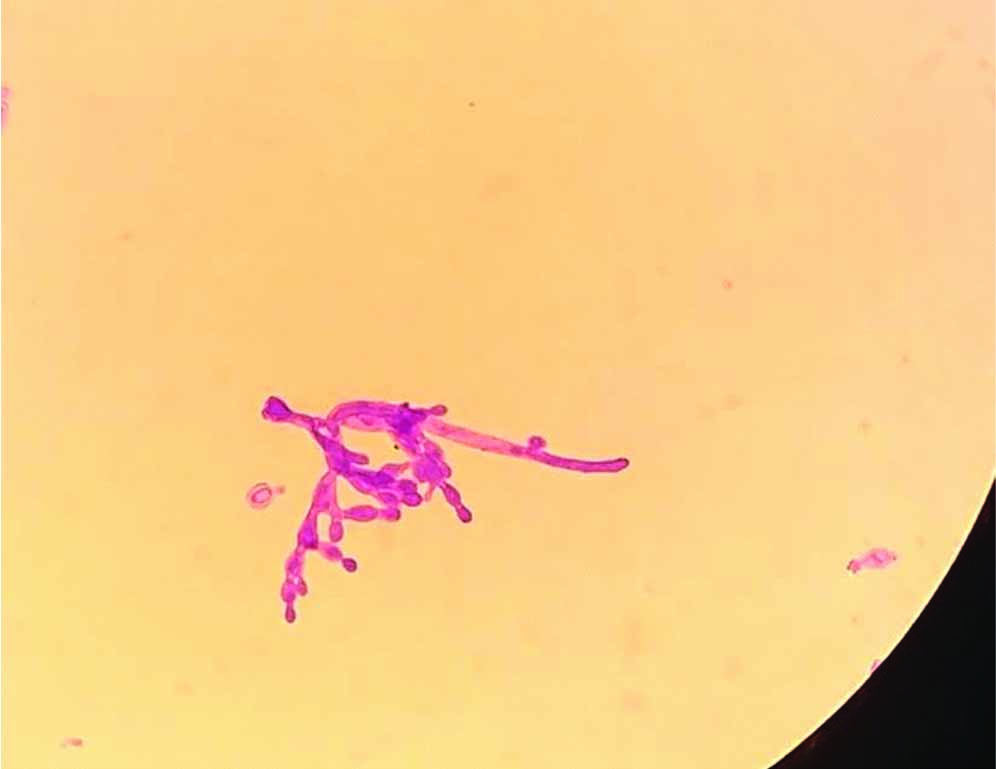
Microscopy of culture-oval blastoconidia in chains of different sizes, arranged along pseudo-hyphae and true hyphae. (Gram stain,X100)
This rare isolate apart from culturing on SDA and Hi-Chrome was also grown on tetrazolium reduction medium (TTZ) which showed pink to white dry rough wrinkled colonies, potato dextrose agar-showed rough non pigmented colonies, oat meal agar- showed rough white wrinkled colonies [Table/Fig-1], corn meal agar-showed dry rough wrinkled growth.
Species Characterisation done with the following biochemical reactions:
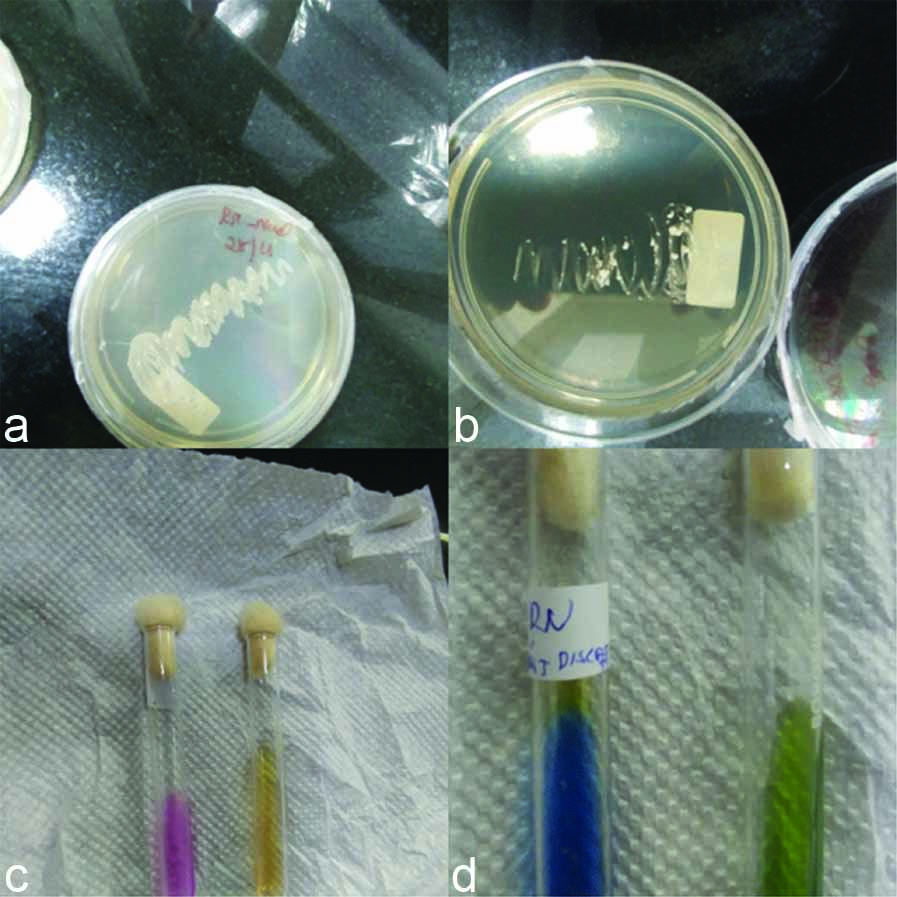
a) Test for citrate utilisation-Simmon’s citrate medium-citrate was utilised (colour changed to blue); b) Test for enzyme production (urease) Christensen’s urea agar-urea not hydrolysed (no colour change) [Table/Fig-3]; c) nitrate not reduced; d) Growth occurred with-SDA with 0.1% cycloheximide, SDA with olive oil overlay, and with 10% NaCl [Table/Fig-3]. Temperature variability-growth was seen at 25°C, 35°C, 37°C. Sugar fermentation and sugar assimilation test was done [Table/Fig-4].
a) Growth with 10% Nacl; b) Growth with actidione; c) Urease test; d) Citrate test.
Showing sugar fermentation and sugar assimilation result of Stephanoascus ciferrii.
| Sugars | Fermentation/growth | Sugars | Assimilation |
|---|
| Glucose | -/+ | Maltose | + |
| Maltose | -/+ | Raffinose | + |
| Lactose | -/- | Xylose | + |
| Galactose | -/+ | Sucrose | + |
| Trehalose | -/+ | Trehalose | + |
| Sucrose | -/+ | Galactose | + |
| Raffinose | -/+ | Lactose | _ |
| Inulin | -/- | Cellobiose | + |
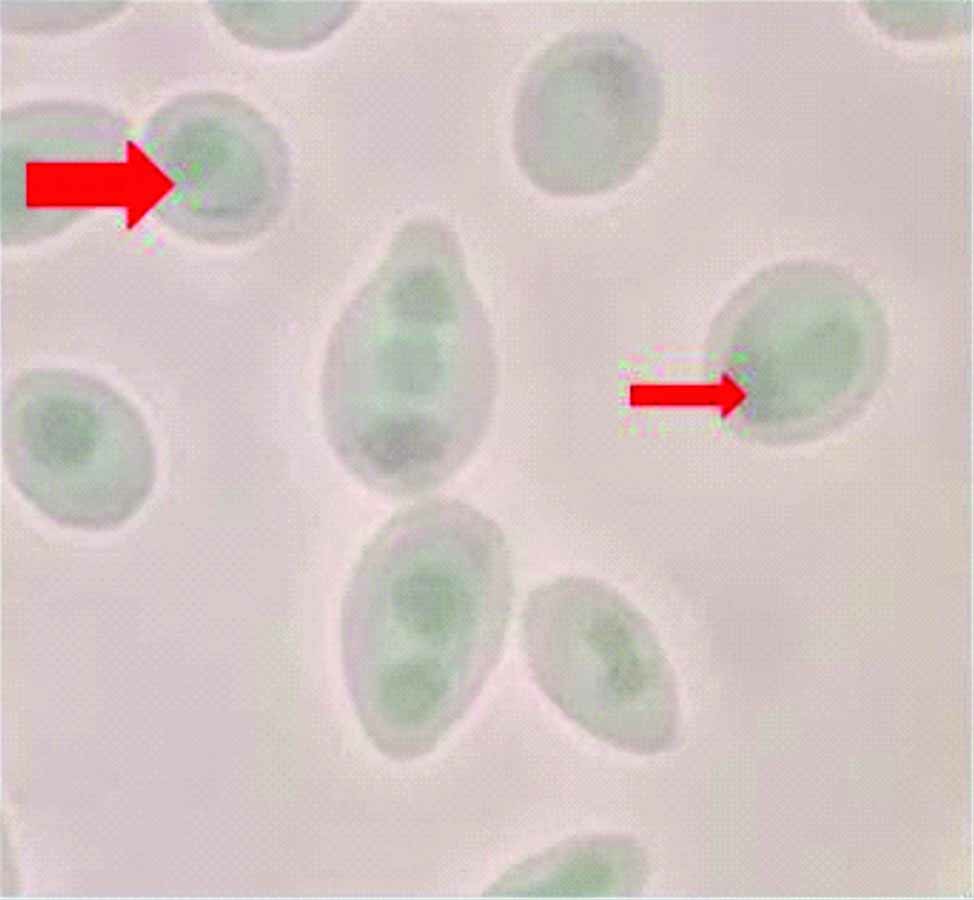
Demonstration of ascospores-Dalmau technique was done with corn meal agar and 1% Tween 80-ascospores with 1-4 asci and typical hat cells were seen [Table/Fig-5]. Slide culture was also done and ascospores were demonstrated by LPCB (Lacto-Phenol Cotton Blue) test. Malt extract agar (Ascospore demonstration medium)-showed ascospores with 1-4 asci and typical hat cells were seen [Table/Fig-5].
Demonstration of ascospores-shows two asci inside ascospores, arrow head shows typical hat shaped ascospores.
Anti-fungal susceptibility testing which was done by disc diffusion method was confirmed by Broth micro-dilution method in accordance with CLSI guidelines M27-A3 (2009). MIC values were obtained. Fluconazole (MIC=>32 μg/mL), Itraconazole (MIC=>16 μg/mL), Voriconazole (MIC=<0.025 μg/mL), Amphotericin-B (MIC=>16 μg/mL), Posaconazole (MIC=<0.025 μg/mL), Caspofungin (MIC=<0.025 μg/mL).
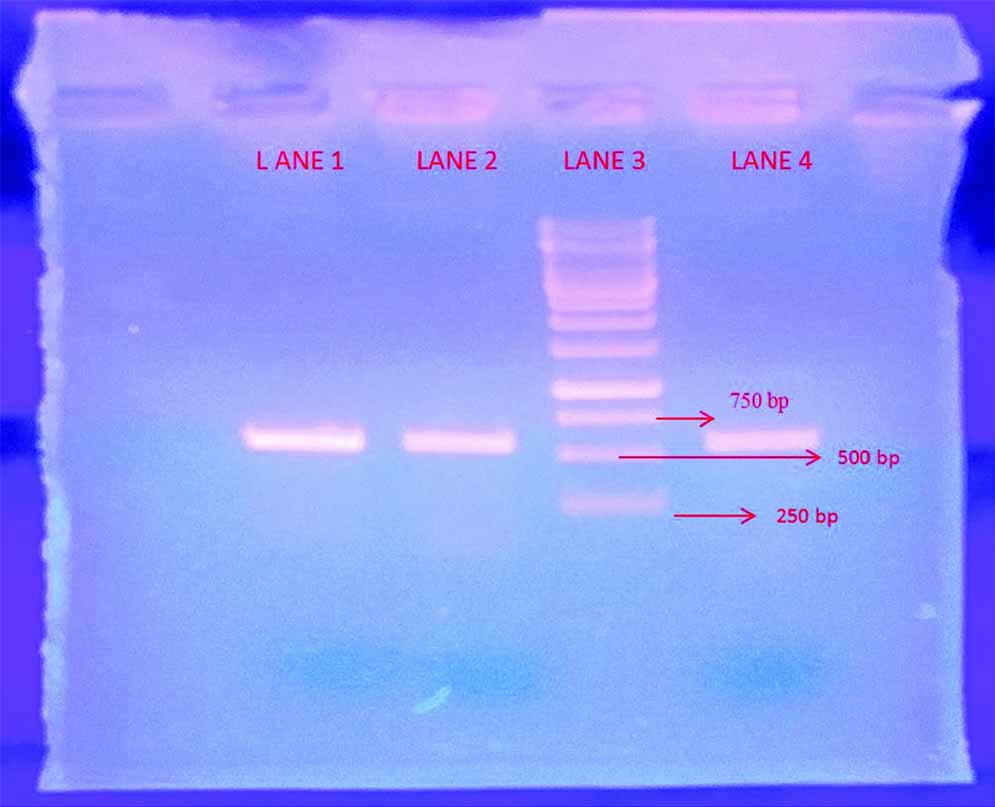
Molecular characterisation was done to confirm the phenotypic identification. DNA was extracted from a 24-hour-old culture on SDA medium by In-house method and Polymerase Chain Reaction (PCR) done using primers its1 (5’ TCC GTA GGT GAA CCT GCG G 3’) and its4 (5’ TCC TCC GCT TAT TGA TAT GC 3’). Band was obtained [Table/Fig-6]. The extracted genes were sequenced using Sangers sequencing. The sequence of genetic material obtained was blasted using NCBI blast and was found to match 100% with CBS (Central Bureau of Fungal Cultures) strain of Trichomonascus ciferrii, thus further confirming our identification of species.
Band obtained from DNA amplification of its 1 and its 4 by PCR.
LANE 3- Ladder
LANE 2, 4- S.ciferrii bands obtained (approx. 550-650 bp)
LANE 1- positive control Candida albicans (approx.550 bp)
Discussion
Candida ciferrii was first described by Kreger van Rij NJ in 1965 [1]. It formed both true hyphae and budding yeast cells and in this respect resembled species of the genera Saccharomycopsis and Trichosporon. As the strains lacked the ability to form fission cells and failed to yield an ascigerous state in either single or mixed cultures, the species was assigned to the genus Candida [2]. After 18s DNA sequencing studies held by Ueda-Nishimura K et al., it was named as Stephanoascus ciferrii, a teleomorph of Candida ciferrii [3]. The Taxonomy of Stephanoascus ciferrii is-Ascomycota, hemi-ascomycetes, saccromycetales: Dipodascaseae [4]. Stephanoascus ciferrii is also called as Trichomonascus ciferrii. It is a rare teleomorph of Candida emerging as a causative agent of many human infections. Stephanoascus ciferrii was first isolated from felines in 2000 [5]. It can cause human infection in immune-compromised host [6,7]. Knowledge of the natural habitat of Candida ciferrii is limited. This species has a strong tendency to become resistant to routinely used anti-fungal agents.
Principal sites of infection reported are ear infections in immuno-competent patients [8], has also been reported from patients with acute myeloid leukaemia [6], immuno-deficient patients, and in cases of superficial and deep mycosis and trophic disorders of leg [9]. Initially, thought to cause only non-invasive infections however it was Gunsilius E et al., who first described the invasiveness of S.ciferrii in immuno-compromised patients [6]. Gradually increasing frequency of invasive fungal infections and the widespread use of empirical antifungal therapy have resulted in the development of drug-resistant fungal strains.
In the recent times there is increase in the rates of infections caused by Candida species, and the diversity of the species causing such infections has also changed. Even more the incidence of isolations and infections associated with uncommon yeast are likely to be underestimated, and they are being evaluated only for case reports.
The prognosis of otitis caused in humans and animals by S. ciferrii is good if it is identified and managed with appropriate anti-fungal agents. Nevertheless, consideration should be given to the opportunistic character of this yeast [7], the immune status of the patient [6] and the resistance displayed to Itraconazole, Fluconazole. The zoonotic potential of this yeast is still unknown.
Conclusion
Candida strains should be identified upto species level and antifungal susceptibility should be performed to choose appropriate drugs to treat the infections caused and reduce the morbidity and mortality due to invasion to the deeper tissues.
Delayed diagnosis may also result in increased mortality rates of invasive fungal infections. Therefore, methods which provide strain-level identification of the Candida species in a rapid and reliable manner are required to initiate the antifungal therapy.
[1]. Kreger-van Rij NJ, Candida ciferrii, a new yeast speciesMycopathol Mycol Appl 1965 26(1):49-52.10.1007/BF020985895876912 [Google Scholar] [CrossRef] [PubMed]
[2]. Smith MT, Van der Walt JP, Johannsen E, The genus Stephanoascus gen. nov.(Ascoideaceae)Antonie van Leeuwenhoek 1976 42(1-2):119-27.10.1007/BF003994551085121 [Google Scholar] [CrossRef] [PubMed]
[3]. Ueda-Nishimura K, Mikata K, Species distinction of the ascomycetous heterothallic yeast-like fungus Stephanoascus ciferrii complex: description of Candida allociferrii sp. nov. and reinstatement of Candida mucifera Kocková-Kratochvílová et SlávikováInt J Syst Evol Microbiol 2002 52(2):463-71.10.1099/00207713-52-2-46311931158 [Google Scholar] [CrossRef] [PubMed]
[4]. De hoogGS, Guarro J, Gene MJ, Figuer as atlas of clinical fungi2nd edition:192-193. [Google Scholar]
[5]. Kano R, Makimura K, Kushida T, Nomura M, Yamaguchi H, Hasegawa A, First isolation of Stephanoascus ciferrii from a catMicrobiology and Immunology 2000 44(8):711-13.10.1111/j.1348-0421.2000.tb02553.x11021402 [Google Scholar] [CrossRef] [PubMed]
[6]. Gunsilius E, Lass-Flörl C, Kähler CM, Gastl G, Petzer AL, Candida ciferrii, a new fluconazole-resistant yeast causing systemic mycosis in immunocompromised patientsAnnals of Hematology 2001 80(3):178-79.10.1007/s00277000025211320905 [Google Scholar] [CrossRef] [PubMed]
[7]. García-Martos P, Ruiz-Aragón J, García-Agudo L, Saldarreaga A, Lozano MC, Marín P, Candida ciferrii in an immunocompromised patientRevistaiberoamericana de micologia 2004 21(2):85-86. [Google Scholar]
[8]. Soki H, Nagase Y, Yamazaki K, Oda T, Kikuchi K, Isolation of the yeast-like fungus Stephanoascus ciferrii by culturing the aural discharge of a patient with intractable otitis media. Case report. KansenshogakuzasshiThe Journal of the Japanese Association for Infectious Diseases 2010 84(2):210-12.10.11150/kansenshogakuzasshi.84.21020420168 [Google Scholar] [CrossRef] [PubMed]
[9]. De Gentile L, Bouchara JP, Le Clec’h C, Cimon B, Symoens F, Chabasse D, Prevalence of Candida ciferrii in elderly patients with trophic disorders of the legsMycopathologia 1995 131(2):99-102.10.1007/BF011028868532062 [Google Scholar] [CrossRef] [PubMed]