Diffuse Nodular Pseudoangiomatous Stromal Hyperplasia causing Bilateral Gigantomastia with Follow-up of 2 Year Post Reduction Mammoplasty
Teena Sleeba1, Jojoe John2, Gigy Raj Kulangara3, Madhu Sudheendran4, Sunita Thomas5
1 Consultant Radiologist, Department of Radiology, Rajagiri Hospital, Aluva, Kochi, Kerala, India.
2 Consultant Pathologist, Department of Pathology, Rajagiri Hospital, Aluva, Kochi, Kerala, India.
3 Consultant Plastic Surgeon, Department of Plastic Surgery, Rajagiri Hospital, Aluva, Kochi, Kerala, India.
4 Consultant Plastic Surgeon, Department of Plastic Surgery, Rajagiri Hospital, Aluva, Kochi, Kerala, India.
5 Consultant Pathologist, Department of Pathology, Rajagiri Hospital, Aluva, Kochi, Kerala, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Teena Sleeba, 1401, Oceanus Maple, Edapally, Kochi-682024, Kerala, India.
E-mail: teenasleeba@gmail.com
Pseudoangiomatous Stromal Hyperplasia (PASH) is an uncommon benign mesenchymal tumour of the breast with no known potential for malignancy. PASH is often detected incidentally or in a background of other breast pathologies. Being hormonally driven, these are most commonly seen in women in the reproductive age group. The size of the PASH varies with small microscopic PASH being much more common than the tumourous or infiltrative varieties. It is uncommon to see PASH presenting as diffuse nodular masses replacing the entire glandular parenchyma symmetrically on both sides. We report a case of a 31-year-old non lactating lady, in whom PASH caused bilateral symmetrical gigantomastia. As PASH has been shown to have a high chance of recurrence, review of literature revealed that most women with PASH-associated gigantomastia undergo mastectomy rather than reduction mammoplasty. We followed up our patient for two years post-reduction mammoplasty with no increase in dimensions of existing lesions or development of new lesions both clinically and on imaging.
Benign mesenchymal tumour, Diffuse nodular mass, Malignancy
Case Report
A 31-year-old non lactating female presented with a two year history of slowly progressive painless breast enlargement. She first noticed small nodules in both the breasts a year after cessation of lactation. Gradually over months, she felt there was an increase both in the number of the lesions and the sizes of her breasts. As these were painless, she kept avoiding a medical consultation. It was only after a dramatic increase in the sizes of both breasts in a span of six months that she was forced to seek medical assistance.
On clinical examination, she had bilateral gigantomastia with breast tissue extending below the umbilicus [Table/Fig-1a]. The breasts were massively enlarged with areas of healing ulcers and dilated veins. She complained of constant upper backache thus making activities of daily living difficult. On examination, severe lordotic deformity of the thoracic spine was seen [Table/Fig-1b]. Clinically innumerable small nodular lesions were palpable and a clinical diagnosis of diffuse fibroadenosis or multiple bilateral fibroadenomas was made.
a) Bilateral gigantomastia with healing ulcers and dilated veins; b) Lordosis of the thoracic region.

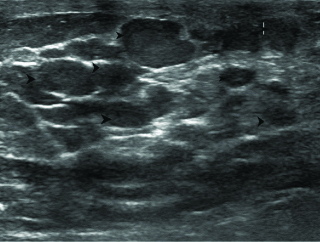
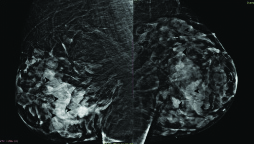
She had no significant past history and work up for autoimmune disorders was negative. There was no family history concerning for breast or gynecological malignancies nor any other female relative with history of gigantomastia. She was non pregnant, non-lactating at the time of presentation and had no history of intake of oral contraceptives. She underwent bilateral mammograms on an analogue system [Table/Fig-2]. The mediolateral views were suboptimal due to the massive breast sizes. Innumerable lesions were seen replacing both breast parenchyma. Ultrasound of the breasts also revealed innumerable small 1-5 cm sized well circumscribed hypoechoic avascular lesions in both breasts [Table/Fig-3]. She subsequently underwent core biopsy with immunohistochemistry which revealed PASH.
Bilateral Mammogram (Craniocaudal views) showing numerous circumscribed lesions replacing breast parenchyma on both sides.

Breast ultrasound showing innumerable 1-5 cm sized ovoid and round circumscribed hypoechoic lesions.
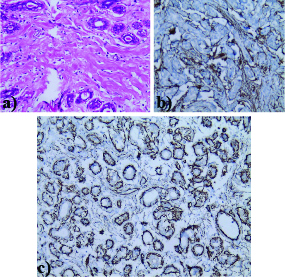
Numerous anastomosing slit-like pseudoglandular spaces were seen on staining with hematoxylin and eosin. These spaces were lined by spindle cells with hyalinized collagen tissue seen in the intervening spaces. The spindle cells showed positive immunoreactivity for myofibroblastic markers like CD34 and SMA [Table/Fig-4a-c]. They were non-reactive for Ki67.
Numerous anastomosing slit-like pseudoglandular spaces lined by spindle cells and separated by thick hyalinized collagen bundles (X100). Spindle cells show positivity for CD34 and SMA respectively.
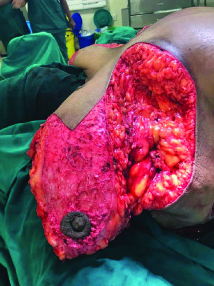
Since the patient was keen on preserving her breasts and was not willing for a mastectomy, she opted for bilateral reduction mammoplasty. She was informed about the high chances of recurrence with PASH prior to surgery. Approximately, two kg parenchyma was removed from the right and 1.8 kg from the left breast respectively. Intraoperatively, innumerable fleshy lesions were seen replacing the parenchyma [Table/Fig-5].
Intraopertive photograph showing numerous fleshy nodular lesions replacing the normal breast parenchyma.
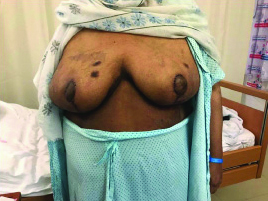
The patient made an uneventful recovery post bilateral reduction mammoplasty [Table/Fig-6]. Apart from PASH, gross specimen failed to reveal any other abnormality. She is on regular follow-up, no recurrence appreciated on both clinical evaluation or on imaging two years after surgery. Mammogram done on a digital system [Table/Fig-7] revealed no further increase in the number or dimensions of existing lesions.
Post bilateral reduction mammoplasty.
Bilateral digital mammograms (Mediolateral oblique views) taken two years later showing residual PASH lesions in both breasts. Clinically and on serial USG, patient remained stable.
Discussion
Tumoural PASH is rare with diffuse tumourous PASH affecting both breasts being even more uncommon. While clinically most often, PASH manifests as solitary small palpable breast masses they are sometimes; the cause for breast enlargement and rarely gigantomastia. Most of the times, when PASH causes gigantomastia it is usually a single large rapidly growing mass. Rarely, they also present as diffuse infiltration of the parenchyma where delineation from normal breast tissue is difficult. Diffuse nodular PASH infiltrating both breasts and causing gigantomastia is extremely rare. We report the case of a 31-year-old lady who had innumerable nodular lesions in both breasts over a span of two years post pregnancy which eventually led to bilateral gigantomastia. Though cases of solitary nodular or multiple nodular PASH had been published, we believe such an extensive nodular variety of PASH, causing bilateral gigantomastia is extremely rare.
PASH is a benign stromal proliferation of the breast most commonly seen occurring in women of reproductive age group [1]. There is a wide variability in presentation clinically as well as on histopathology. On microscopic evaluation, PASH has been seen as tiny foci or incidentally detected lesions in association with other mainly benign proliferative breast pathologies such as fibroadenoma, stromal fibrosis, phyllodes, and atypical ductal or lobular hyperplasia [2-4].
The most postulated aetiological theory is hormonal stimulation of mammary myofibroblasts, particularly by progesterone. The main clinical differential diagnosis is a fibroadenoma or phylloid tumour while histologically they need to be diffrentiated from a low-grade angiosarcoma.
Typically PASH is seen as areas of stromal hyperplasia with empty slit-like channels which are lined by spindle cells. Immunoreactivity with myofibroblastic markers like SMA, vimentin and CD34 is seen. Vascular endothelial markers are negative in PASH diffentiating it from tumours like a low grade angiosarcoma [4]. A pubmed search of PASH causing gigantomastia revealed only eight cases. Though, less than 20 cases of diffuse infiltrative PASH have been described in literature, this variety causing gigantomastia is commoner than the diffuse nodular variety where the parenchyma was replaced by innumerable nodular PASH lesions [5-7]. The only other case we came across in literature with similar presentation was reported by Samaila MO et al., who also noted that diffuse nodular variety of PASH occurring symmetrically was a rarity [8]. They reported rapid breast enlargement in a patient who initially underwent excision of bilateral breast lumps. She then had subsequent gigantomastia within a year of surgical excision and had to undergo bilateral mastectomy after she presented with multiple non-healing venous ulcers and peau d’orange in both breasts.
However, in our patient despite similar presentation and morbidity due to bilateral nodular PASH, the patient was unwilling to undergo bilateral mastectomy and opted only for reduction mammoplasty. Prior to the procedure, we explained the high recurrence rates with PASH and also the need to undergo subsequent mastectomy, once the present procedure failed.
The treatment for single nodular PASH is usually surgical excision, though recurrences have been recorded. Known to have a high recurrence rate of approximately 22% [8] and hence despite its benign nature, PASH causing unilateral or bilateral gigantomastia have almost always been managed with mastectomy and subsequent reconstruction [6,9,10]. Diffuse multiple lesions with incomplete excision are often associated with rapid growth [6].
We wish to highlight this unique case where our patient has been stable for two years after undergoing reduction mammoplasty for diffuse nodular PASH. The number and dimensions of the largest PASH lesions are same for two years on imaging with Mammogram and USG, there has been no change in her bra size since operation, and she has been able to carry on with activities of daily living like prior to the onset of gigantomastia. A written consent was taken from the patient prior to publication of this case.
Conclusion
PASH is a benign entity whose occurrence as clinically palpable lesions is uncommon. It is also an extremely rare cause for massive breast enlargement. We believe this is the second reported case of gigantomastia secondary to diffuse nodular PASH affecting both breasts. Conservation surgery for such extensive disease is uncommon. Moreover, follow-up for cases who underwent conservation surgery is lacking. Our patient chose to have reduction mammoplasty with no disease recurrence appreciated on a two year follow-up suggesting the possibility of conservation surgery as a first option especially in young women even with such extensive disease.
[1]. Vuitch MF, Rosen PP, Erlandson RA, Pseudoangiomatous hyperplasia of mammary stromaHum Pathol 1986 17:185-91.10.1016/S0046-8177(86)80292-1 [Google Scholar] [CrossRef]
[2]. Bowman E, Oprea G, Okoli J, Gundry K, Rizzo M, Gabram-Mendola S, Pseudoangiomatous stromal hyperplasia (PASH) of the breast: A series of 24 patientsBreast J 2012 18:242-47.10.1111/j.1524-4741.2012.01230.x22583194 [Google Scholar] [CrossRef] [PubMed]
[3]. Powell CM, Cranor ML, Rosen PP, Pseudoangiomatous stromal hyperplasia (PASH). A mammary stromal tumour with myofibroblastic differentiationAm J Surg Pathol 1995 19:270-77.10.1097/00000478-199503000-000047872425 [Google Scholar] [CrossRef] [PubMed]
[4]. Virk RK, Khan A, Pseudoangiomatous stromal hyperplasia: An overviewArch Pathol Lab Med 2010 134:1070-74. [Google Scholar]
[5]. Yoo K, Woo OH, Yong HS, Kim A, Ryu WS, Koo H, Fast-growing pseudoangiomatous stromal hyperplasia of the breast: Report of a caseSurg Today 2007 37:967-70.10.1007/s00595-007-3540-617952527 [Google Scholar] [CrossRef] [PubMed]
[6]. Ryu EM, Whang IY, Chang ED, Rapidly growing bilateral pseudoangiomatous stromal hyperplasia of the breastKorean J Radiol 2010 11:355-58.10.3348/kjr.2010.11.3.35520461190 [Google Scholar] [CrossRef] [PubMed]
[7]. Dai H, Connor C, Cui W, Gatewood J, Fan F, Bilateral diffuse tumourous pseudoangiomatous stromal hyperplasia: A case of bilateral mastectomy in a 29-year-old womanCase Rep Pathol 2014 2014:25060810.1155/2014/25060825544925 [Google Scholar] [CrossRef] [PubMed]
[8]. Samaila MO, Aliyu HO, Yusufu LM, Abdullahi S, Concurrent giant tumoural pseudoangiomatous stromal hyperplasia necessitating bilateral mastectomyAnn Afr Med 2018 17(2):82-85.10.4103/aam.aam_27_1729536962 [Google Scholar] [CrossRef] [PubMed]
[9]. Krawczyk N, Fehm T, Ruckhäberle E, Mohrmann S, Riemer J, Braunstein S, Bilateral diffuse Pseudoangiomatous Stromal Hyperplasia (PASH) causing gigantomastia in a 33-year-old pregnant woman: Case reportBreast Care (Basel) 2016 11(5):356-58.10.1159/00045086727920630 [Google Scholar] [CrossRef] [PubMed]
[10]. Oppenheimer AJ, Oppenheimer DC, Fiala TG, Noori S, Pseudoangiomatous stromal hyperplasia: A rare cause of idiopathic gigantomastiaPlast Reconstr Surg Glob Open 2016 4(1):e59310.1097/GOX.000000000000057227104092 [Google Scholar] [CrossRef] [PubMed]