The administration of systemic chemotherapy in order to shrink the size of the tumour before surgical resection is termed as NACT. It was initially described for advanced inoperable carcinomas, but is now being increasingly used for operable cancers as well. The idea is to improve surgical outcome, reduce risk of distant metastasis, achieve tumour shrinkage, downstage the disease, evaluate the effectiveness of systemic therapy and prolong overall disease free survival. Histopathological tumour regression is considered as the gold standard for the assessment of treatment response in many solid malignancies [1]. The distinctive advantage of Neoadjuvant Therapy (NAT) in breast carcinoma is the ability of the patients to undergo breast conserving surgeries and achieve negative axillary lymph node status.
Studies have shown that the patients who do not have any evidence of residual tumour, post NACT, correspond with longer disease free and overall survival [2]. The pathologists are increasingly encountering breast carcinoma specimens which have received NACT due to current trends in breast cancer management [3]. Moreover, standard guidelines for assessment of specimens following NACT has not been clearly described [4]. In the present study, we aim to study various histo-morphological features in breast carcinomas following NACT and evaluate the utility of the Chevallier grading system in assessment of histological response.
Materials and Methods
The present study was a prospective study that included 22 patients who had been diagnosed with breast carcinomas and had undergone NACT who were referred to a tertiary care hospital in Southern India between November 2013 and December 2014. The Institutional ethics committee clearance was obtained prior to commencement of study. All patients with breast carcinomas who had previously received NACT followed by Modified Radical Mastectomy (MRM) having a previous biopsy diagnosis prior to NACT were included in the present study. The patients who did not received NACT, incisional biopsies, and those cases where a biopsy prior to NACT administration was unavailable were excluded from the present study.
In each case, a detailed history and clinical examination, radiological investigations and previous biopsies were recorded from the medical records of the patients. The resection specimens obtained after MRM following NACT were studied as per standard protocol. The specimens were fixed in 10% formalin, grossed and sections were taken from the lesion and the lymph nodes. The tissue bits were processed and embedded in paraffin. The paraffin sections were stained by Haematoxylin and Eosin (H&E) stain and studied under the microscope. A detailed study of the histopathological specimens following chemotherapy were evaluated for histo-morphological changes. The tumour cells were evaluated for nuclear features such as karyorrhexis, pyknosis and karyolysis. The cytoplasmic features studied included vacuolation of cytoplasm and foamy cell change. The stroma was examined for host response such as inflammatory reaction, giant cell reaction and hyalinization. The pathological response was evaluated by Chevallier grading system [5].
Pathological response grading was done as follows:
Pathological Complete Response (pCR)- No residual invasive carcinoma or DCIS in breast or lymph nodes.
Pathological Partial Response (pPR)- Presence of residual invasive carcinoma exhibiting stromal alterations.
Pathological No Response (pNR)- Little change in appearance or original carcinoma. The results thus obtained were analysed and tabulated.
Results
A total of 22 cases of breast carcinomas were included in the present study. The age of the patients ranged from 27 to 70 years (mean=49.5).
All the patients in the study presented clinically with a palpable breast lump. In the present study, both the left and the right breast were encountered with equal frequency. The most commonly affected site was the upper outer quadrant (n=5). The skin changes commonly encountered were ulceration, fungation and hyperpigmented nodule.
A previous biopsy report were available for all the cases of which a diagnosis of infiltrating ductal carcinoma was most commonly documented (n=20), followed by one case of infiltrating lobular carcinoma and infiltrating papillary carcinoma respectively.
Evaluation of ER, PR, HER2/neu status prior to NACT was documented in five cases. Majority were ER, PR positive and HER2/neu negative.
All the cases in the present study received chemotherapy and the number of cycles ranged from 3 to 11 cycles.
Residual tumour following NACT was present in 17 cases of which a grey white infiltrative growth was the most commonly encountered on gross finding (n=13), followed by two cases of fungating growth and one case of cyst and sclerosis respectively. The gross findings following NACT are depicted in [Table/Fig-1].
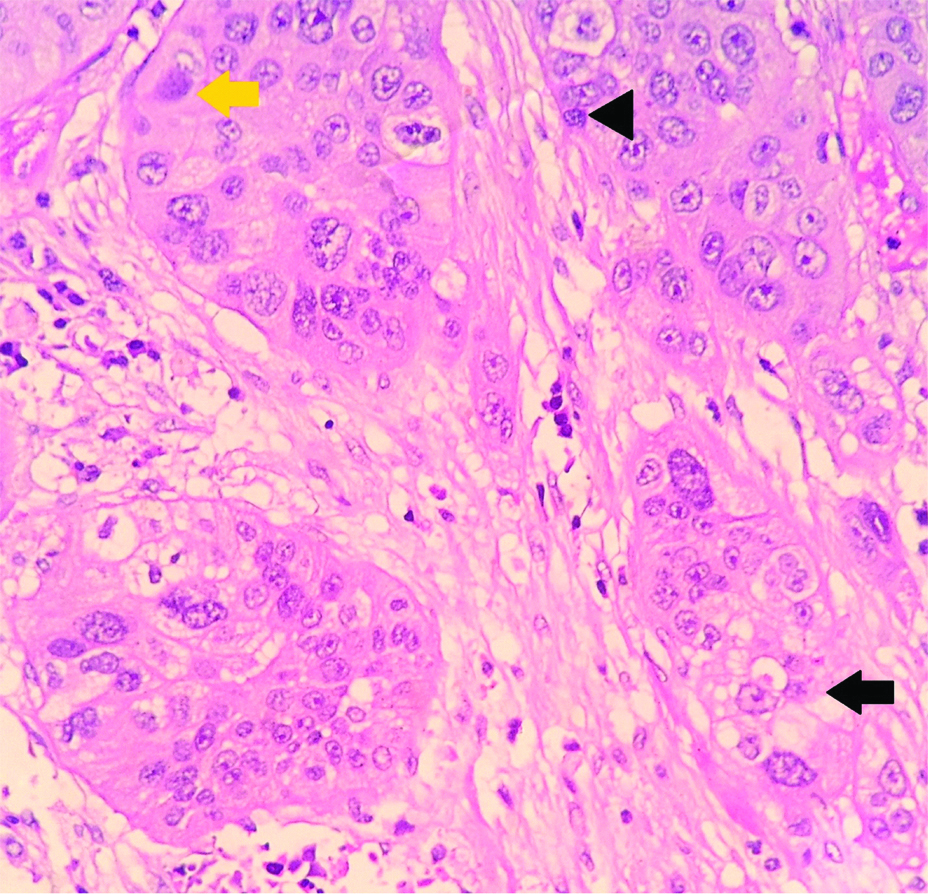
Nuclear features following neoadjuvant chemotherapy in breast carcinomas including karyolysis (yellow arrow), pyknosis (Black arrowhead) and karyorrhexis (Black arrow) (H&E, X400).
In the cases with pCR, the finding of sclerosis (n=5) was common.
Majority of the residual tumours were infiltrating ductal carcinomas (n=15), followed by infiltrating lobular carcinoma (n=1) and infiltrating papillary carcinoma (n=1). It was observed that there was no change in histological type after administration of NACT.
Of the cases studied, an SBR grade of 2 was noted in 13 cases whereas SBR grade of 3 was noted in four cases.
The various patterns of arrangement of residual tumour were tubules, sheets, nests, papillae, cords, trabaculae, comedo and cribriform.
Necrosis was observed in 16 cases. All of the cases showing pCR to NACT showed necrosis. Among the nuclear alterations following NACT, the most commonly encountered was pyknosis (n=16 cases). The other nuclear alteration observed were karyorrhexis (n=14) and karyolysis (n=14) [Table/Fig-1].
Vacuolation was a commonly associated cytoplasmic alteration seen in 15 cases.
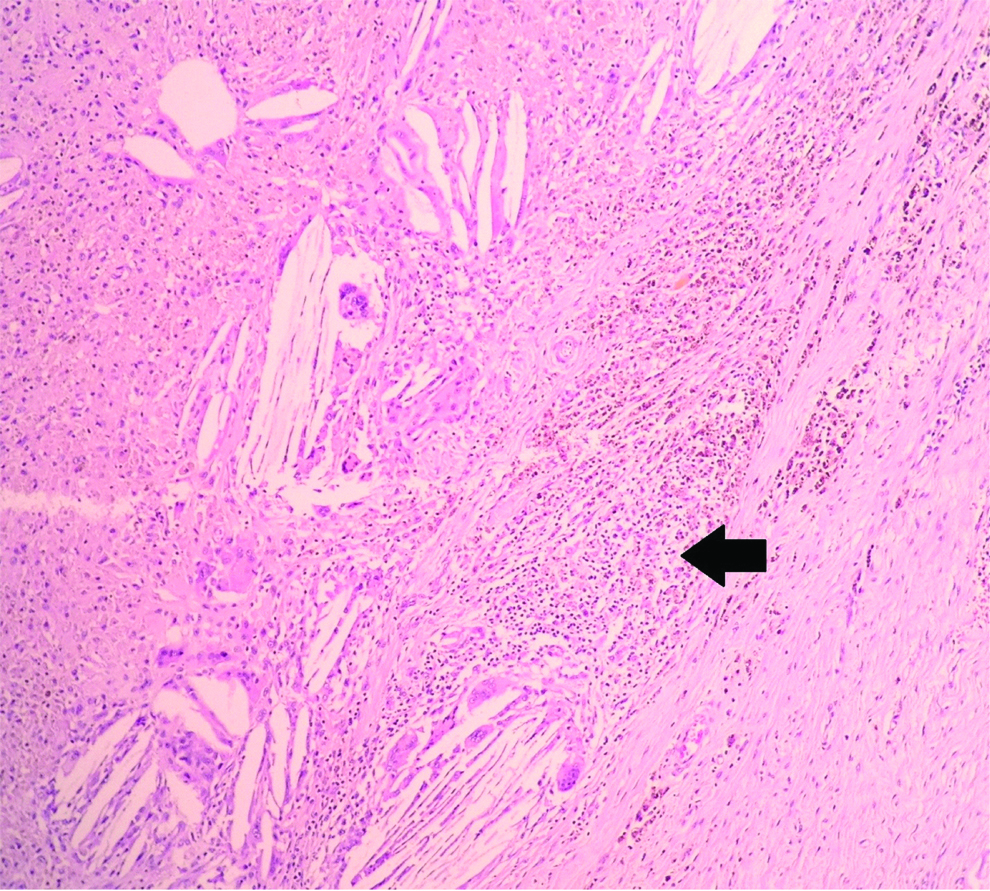
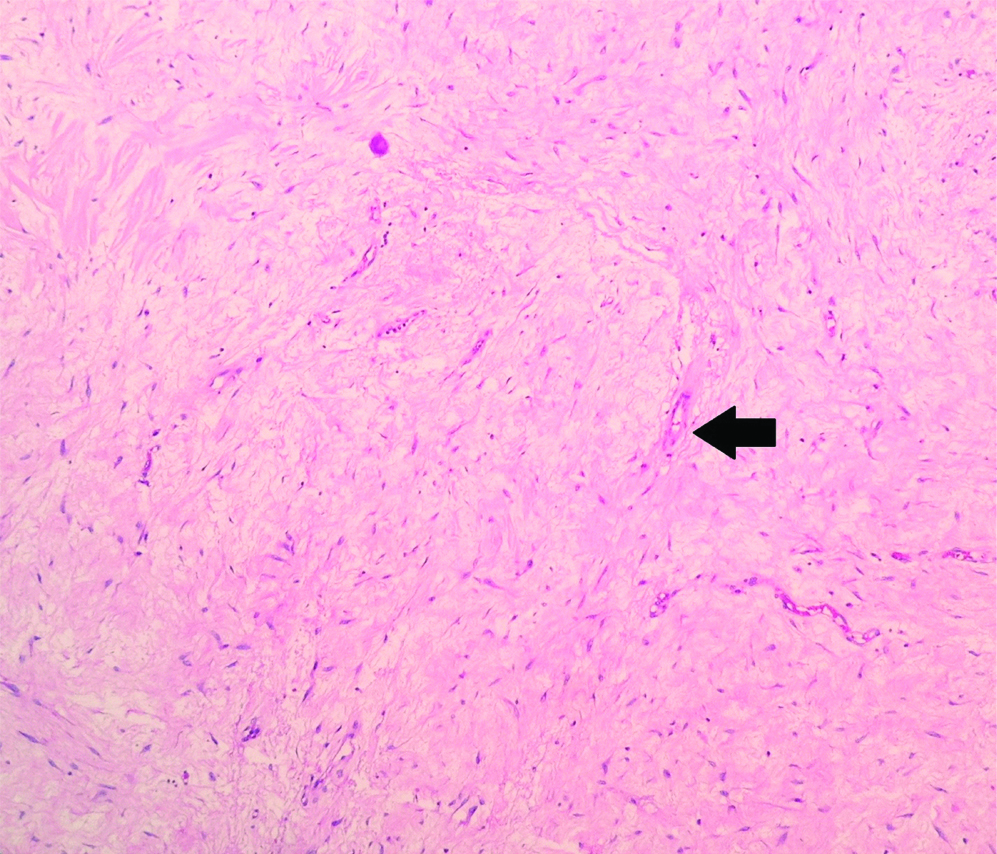
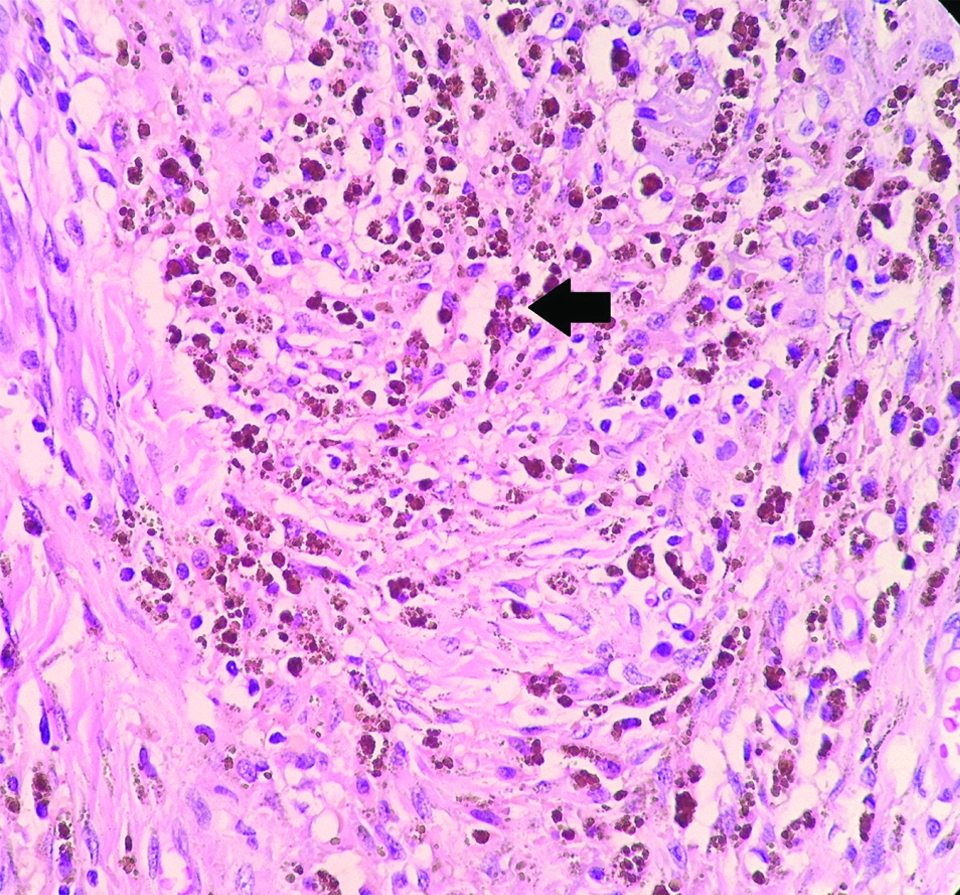
The consistently found stromal alteration were lymphoplasmacytic infiltrate (n=19), followed by hyalinisation (n=17), collection of foamy macrophages (n=5), fibrosis (n=5), haemosiderin laden macrophages (n=5), giant cell formation (n=5) [Table/Fig-2,3 and 4].
Stromal alterations following neoadjuvant chemotherapy in breast carcinomas showing lymphoplasmacytic infiltrate (black arrow). (H&E, X200).
Stromal alterations following neoadjuvant chemotherapy in breast carcinomas showing sclerosis (black arrow) (H&E, X400).
Stromal alterations following neoadjuvant chemotherapy in breast carcinomas showing haemosiderin laden macrophages (black arrow) (H&E, X400).
Lymphovascular invasion was observed in 13 cases while lymph node metastasis was observed in 17 cases.
Discussion
NACT is the delivery of systemic chemotherapeutic agents prior to definitive surgery. Many studies have shown the benefits of NACT to be manifold [2]. The response to a systemic agent can be studied and a patient can be assessed accordingly. Those patients showing a pCR may have a long term disease free interval. As NACT is being increasingly adapted for treatment of early stage malignancies, Pathologists need to be aware of these varied constellation of NACT induced changes [6].
Clinical trials have shown excellent correlation between response to NAT and disease free overall survival in patients with breast carcinomas.
A total of 22 cases of breast malignancies that received NACT were analysed in the present study. In the present study, the age of the patients ranged from 27-70 years (median=49.5) similar to a study by Carey et al., [7]. The majority of the patients in their study received NACT while a proportion of cases received radiotherapy. In contrast, all the patients in the present study received only chemotherapy. According to the authors, there was no accurate and reliable response classification system, while most resorted to pCR as a tool for assessing response. They found that the revised AJCC (2003) classification was effective in assessing response. The other response systems recommended by the authors was the response used by the Milan Cancer institute and a method devised by the authors themselves in a previous study. They were of the opinion that the pCR albeit prognostic, was limited due to the small number of patients who achieved the end result [8-10].
In the present study, a thorough clinical assessment of primary breast lesion and axillary lymph nodes by physical examination was performed in each case, supplemented by relevant radiological and pathological study. A few of the researchers are of the opinion that physical examination and mammography is essential, others feel that sonography correlates best with pathological study [11].
Evaluation of tumour markers such as ER, PR and HER2/neu prior to NACT was available in five cases of which majority were ER, PR positive while all the tumours were HER2/neu negative. According to some studies, by and large the hormonal status remains the same following NACT; however some types of therapy may induce a change in the tumour marker expression [2].
Studies have shown different variables to be prognostically significant. The amount of residual tumour in specimens following NACT has been found to be an excellent prognostic indicator [6]. The number of positive lymph nodes in a surgical specimen with prior NACT and the number of chemotherapy cycles given were also independent prognostic indicators [12]. In the present study, the number of cycles of chemotherapy ranged from 3-11 in number. A pCR was seen in those cases which had received 4 or more cycles while a pNR was seen in those cases with 3 cycles of chemotherapy. Majority of the cases showed pNR following NACT.
In all of the cases showing pCR to chemotherapy, sclerosis was the most common finding. Residual tumour following pNR to NACT was noted in 16 cases. Of these, the most common gross finding was an infiltrative growth followed by fungating growth, cyst and sclerosis. Majority of the residual tumours were infiltrative ductal carcinoma with an SBR grade of 2. These findings were similar to a similar study by Sethi D et al., [6].
Necrosis was observed in 16 cases. All the cases showing pCR to NACT showed necrosis. According to some studies, histopathological measurement of tumour necrosis constituted an important prognostic variable in patients with osterosarcoma who received NACT [13]. A study done by Bonadonnna et al., [8]., has shown that following administration of NACT, cellularity of tumour mass shows significant reduction. However, this change is highly variable among different patients.
Following NACT, a constellation of histo-morphological changes were noted in the present study. Previous studies have similarly described several nuclear and cytoplasmic alteration following NACT such as nuclear enlargement, nuclear skrinkage, nuclear pyknosis, nuclear vacoulation, necrosis, cytoplasmic vacoulation and degenerative changes, loss of architecture, dyscohesion, shrinkage of tumour cells, karyorrhexis, karyolysis [5,14-16].
In the present study, the most commonly encountered nuclear alterations following NACT was pyknosis while others such as karyorrhexis and karyolysis were observed to a lesser extent. Vacuolation was the most commonly seen cytoplasmic alteration along with foamy cell change. The consistently found stromal alteration was lymphoplasmacytic infiltrate, followed by hyalinisation, collection of foamy macrophages, fibrosis, haemosiderin laden macrophages, giant cell formation. In a similar study, the authors studied stromal changes such as fibrosis, elastosis, collagenisation, hyalinisation, microcalcification, neovascularisation, lymphocytic infiltrate and giant cell reaction of which giant cell reaction significantly correlated to all types of tumour responses [5].
According to some studies, lymph node status is an important for prognosis in patients who have received NACT with patients who have achieved complete response in both breast as well as axillary nodes have been shown to have a better disease free overall survival [2].
Lymphovascular invasion was observed in 13 cases while lymph node metastasis was observed in 17 cases. Studies by Dershaw et al., concluded that NACT was a significant independent parameter for a reduced lymph node yield following an axillary dissection [11].
Limitation
The limitations of the study include small sample size and unavailability of follow-up data. Furthermore, data on clinical staging of the tumour prior to commencement of NAT as well as following NAT was unavailable.
Conclusion
Pathologists need to be aware of the myriad of histo-morphological features that may be seen in specimens following radical surgery. Sclerosis was the most common gross finding and necrosis, pyknosis, cytoplasmic vacuolation and lymphoplasmacytic infiltrate were the common histological finding in cases with pCR and in cases showing moderate and poor response to NACT.