Efficacy and Safety of High-Flow Nasal Cannula Oxygen Therapy in Moderate and Severe Bronchiolitis
Sunil V Kapur1, Jitendra S Oswal2, Bhakti Sarangi3
1 Senior Resident, Department of Pediatrics, Bharati Vidyapeeth University Medical College and Hospital, Pune, Maharashtra, India.
2 Professor and Deputy Medical Director, Department of Pediatrics, Bharati Vidyapeeth University Medical College and Hospital, Pune, Maharashtra, India.
3 Assistant Professor, Department of Pediatrics, Bharati Vidyapeeth University Medical College and Hospital, Pune, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Jitendra S Oswal, Professor and Deputy, Medical Director, Department of Pediatrics, Bharati Vidyapeeth University Medical College and Hospital, Pune, Maharashtra, India.
E-mail: jsoswal@gmail.com
Introduction
Heated humidified High-Flow Nasal Cannula therapy (HFNC) is a non-invasive form of oxygen delivery, in which oxygen supports respiration by reducing nasopharyngeal dead space, decreasing airway oedema, enhancing ciliary activity and providing positive airway pressure.
Aim
To evaluate efficacy and safety of HFNC oxygen therapy in children with moderate and severe bronchiolitis.
Materials and Methods
The present prospective study was carried out in PICU of a tertiary care hospital over a period of 24 months. Twenty two children between the ages of 2 months to 2 years who were previously healthy and diagnosed with moderate and severe bronchiolitis were included. A standard protocol was used for all the children with regards to the initiation, monitoring and weaning from HFNC. Outcome parameters measured were Heart Rate (HR), Respiratory Rate (RR), oxygen saturation (SpO2), Arterial Blood Gases (ABG), hours of therapy, and failure of HFNC oxygen therapy. Parameters were compared using two tailed test.
Results
Out of 22 children, 15 were male and 7 were female with M:F ratio 2:1. Eight children had moderate bronchiolitis and 14 children had severe bronchiolitis. The mean age (±SD) was 7.18±4.48 months. The mean baseline HR, RR, SpO2, PaCO2, PaO2 levels were 158.50±35.19 beats/min, 68.64±10.72/min, 88.68±2.12%, 31.23±6.12 mmHg, 122.73±44.94 mmHg respectively. At the end of one hour of HFNC oxygen therapy, mean HR was 151.59±14.61 beats/min, RR 59.32±9.61/min, SpO2 99.59±0.59%, PaCO2 30.99±6.16 mmHg and PaO2 125.71±37.12 mmHg. There was statistically significant improvement (p<0.05) in the work of breathing as indicated by fall in mean HR and RR along with increase in mean SpO2 level after one hour of HFNC oxygen therapy. This improvement was consistently seen till the end of the study. The mean hours for which HFNC oxygen therapy was required was 43.27±16.31 hours. One child failed HFNC oxygen therapy and required invasive ventilation. There were no serious adverse events.
Conclusion
HFNC oxygen therapy significantly decreases the work of breathing and improves oxygen saturation in moderate and severe bronchiolitis.
Bronchiolitis, Oxygen saturation, Respiratory rate
Introduction
Bronchiolitis is an acute inflammation of the bronchioles that is usually caused by a viral infection in a child <2 years of age [1]. Shi T et al., estimated that globally in 2015, 33·1 million episodes of bronchiolitis resulted in about 3·2 million hospital admissions, and 59, 600 in-hospital deaths in children younger than 5 years [2]. The most common etiological causes are respiratory syncytial virus, influenza virus, parainfluenza virus, adenovirus, corona virus, human metapneumovirus, and mycoplasma pneumoniae [3]. The current approach of management of bronchiolitis is focused on oxygen therapy for hypoxia, respiratory support and the maintenance of hydration [4,5]. Traditionally, oxygen is provided at 100% concentration via low flow nasal prongs as a dry gas which is not heated. However, a recent randomised control study in 177 children, aged 1 month to 5 years revealed that HFNC was more effective in children presenting with acute respiratory distress compared to conventional oxygen therapy [6]. The inspired oxygen concentration (FiO2) can vary from 21% to 100%; therefore, giving greater ability to titrate the concentration of oxygen delivered [6]. It is still not clear whether use of HFNC oxygen therapy will mitigate the use of invasive ventilator supports, such as Continuous Positive Airway Pressure (CPAP) and mechanical ventilation. HFNC was initially used to treat preterm infants as an alternative to CPAP [7,8] but it has recently become very popular in paediatrics and increasing in adults (especially in ICU settings) [9-12].
HFNC has been extensively studied in children with bronchiolitis in US and other developed countries [9,10]. These studies can’t be applied directly on Indian population, as racial differences, and disease pattern/severity is different as compared to developed countries. There is limited clinical evidence of HFNC in infants and young children other than small observational studies which were conducted in older children [12]. Hence this study was conducted with the aim of evaluating efficacy and safety of HFNC oxygen therapy in children with moderate and severe bronchiolitis.
Materials and Methods
The present prospective study was conducted in a PICU of a tertiary care hospital in Western Maharashtra over a period of 24 months (1st September 2015 to 31st August 2017) with a sample size of 22 children. Sample size was based on literature review [8, 9] and hospital admission data for bronchiolitis. The ethical clearance (approval number BV/DU/MC/2051/15-16) was obtained from institutional ethics committee. The written informed consent from either of parents was obtained. The previously healthy children between ages of 2 months to 2 years diagnosed with moderate and severe bronchiolitis who were not maintaining saturation (SpO2 <95%) on 31% venturi for 30 minutes were included in the study. Children with congenital abnormalities of upper and lower respiratory tract, lobectomy, congestive cardiac failure and neuromuscular disorders were excluded from the study. A standard protocol was used for all the children with regards to the initiation, monitoring and weaning from HFNC [Table/Fig-1]. The HFNC system (Fisher & Paykel Healthcare Airvo 2™) consists of a humidifier and a continuous flow circuit. Outcome parameters measured were HR, RR, SpO2, PaO2, PaCO2, hours of therapy, failure of HFNC oxygen therapy and adverse events. The HR, RR, SpO2 were monitored hourly until HFNC therapy was completed or HFNC failed. ABG analysis was done at hours 1, 3, 12, 24, 48 and completion of HFNC therapy. Additional ABG analysis was done as and when needed.
Standard protocol used for all children with regards to initiation, monitoring and weaning from HFNC.
| For the study:Bronchiolitis is defined as a seasonal viral illness characterised by fever, nasal discharge, and dry, wheezy cough in children less than 2 years of age.Mild Bronchiolitis:Normal ability to feed, little or no respiratory distress with no oxygen requirement and saturations above 95% in room air.Moderate Bronchiolitis:Increase work of breathing during feeding, feeds may decrease but total intake is more than 50% of normal, mild to moderate respiratory distress with some chest wall retractions & nasal flaring, and oxygen saturations 90-95% in room air.Severe Bronchiolitis:Reluctant to feed with intake less than 50% of normal, moderate to severe respiratory distress with marked chest wall retractions, nasal flaring and grunting +/- apnoeic episodes and Oxygen saturations less than 90% in room air.Baseline HR, RR, SpO2 and ABG was documented.Classify:Mild Bronchiolitis: Exclude from study.Moderate and Severe Bronchiolitis: Start oxygen on venturi. If not maintaining saturation (SpO2 <95%) on 31% venturi for 30 mins, then commence HFNC therapy.Initiation:HFNC was commenced at 2 L/kg/min (<10 kg), and for greater than 10 kg, additional 0.5 L/Kg/min for every Kg. FiO2 was started at 60%, and was titrated every 5 mins to maintain saturation ≥95% for 12 hours (Min FiO2 40%).Monitoring:HR, RR and SpO2 was monitored hourly until HFNC therapy was completed or HFNC failure.ABG was repeated at Hours 1, 3, 12, 24, 48 and completion of HFNC therapy. In addition, ABG was repeated as and when needed.Weaning:Reduce FiO2 to 40%.Decrease flow rate by 1 L/Hr.When flow rate is tapered to 5 L/Hr and saturation maintained at ≥95%-Discontinue HFNC which was considered as completion of HFNC therapy.End Point of the study:Completion of HFNC therapy or failure of HFNC was defined as need of endotracheal intubation on HFNC oxygen therapy. |
Statistical Analysis
The collected data were coded and entered into Microsoft excel sheet. Statistical analysis was done using SPSS version 20.0 (Armonk, NY: IBM Corp). The continuous variables were presented as mean±standard deviation. The categorical variables were presented as absolute numbers and percentages. A two tailed test with p-value<0.05 was considered significant.
Results
Out of 22 children enrolled in the study, 15 (68%) were male and 7 (32%) were female. The male to female ratio was 2:1. The mean age (±SD) of children enrolled in the study was 7.18±4.48 months. Eight children had moderate bronchiolitis and 14 children had severe bronchiolitis. Failure of HFNC requiring mechanical ventilation was not an exclusion criteria and data was included in the final analysis.
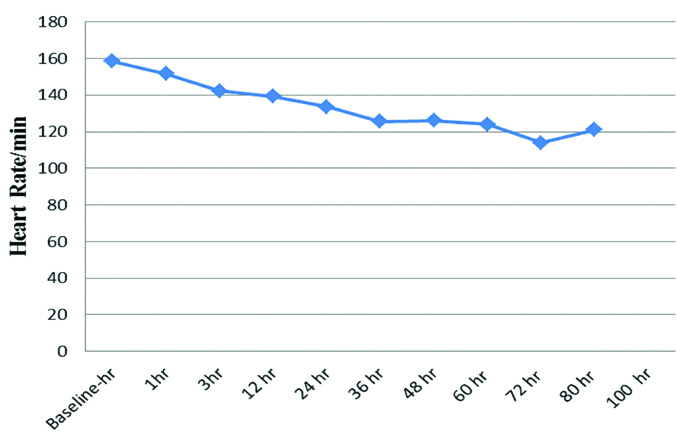
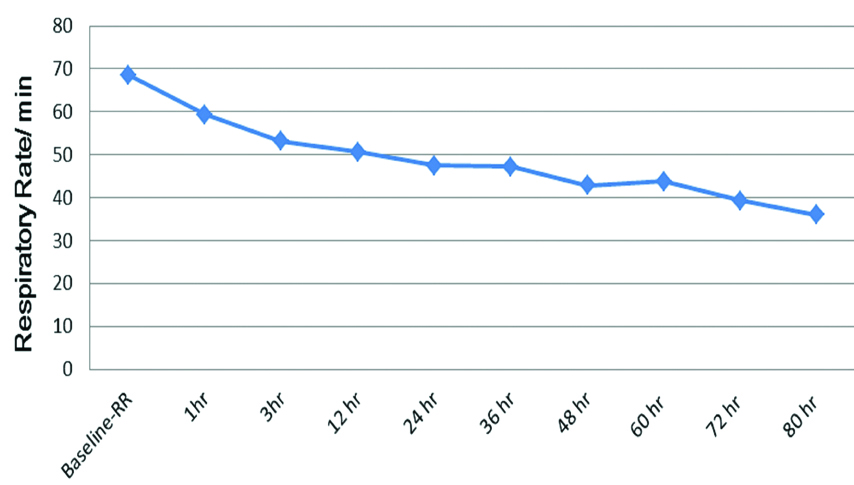
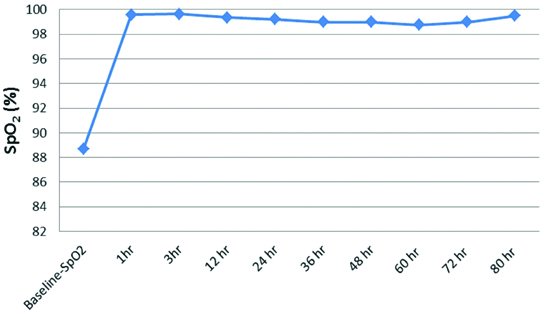
The baseline mean HR, RR, SpO2, PaCO2, PaO2 levels were 158.50±35.19 beats/min, 68.64±10.72/min, 88.68±2.12%, 31.23±6.12 mmHg, 122.73±44.94 mmHg respectively [Table/Fig-2]. There was a decline in HR from baseline 158.50±35.19 beats/min to 151.59±14.61 beats/min at the end of one hour of HFNC therapy (p<0.05). The decline in HR remained persistent throughout the end of the study (80 hours) which was statistically significant (p<0.05) [Table/Fig-3]. There was a decline in RR from baseline 68.64±10.72/min to 59.32±9.61/min at the end of one hour of HFNC oxygen therapy (p<0.05). The decline in RR remained persistent throughout the end of the study which was statistically significant (p<0.05) [Table/Fig-4]. The baseline mean SpO2 88.68±2.12% improved to 99.59±0.59% at the end of one hour of HFNC oxygen therapy (p<0.05). The improvement in SpO2 was persistent throughout the end of the study which was statistically significant (p<0.05) [Table/Fig-5]. There was a slight decline in PaCO2 from baseline 31.23±6.12 mmHg to 30.99±6.16 mmHg at the end of one hour of HFNC oxygen therapy (p=0.760). PaCO2 levels remained similar throughout the study (p=0.129). The mean PaO2 improved from baseline 122.73±44.94 mmHg to 125.71±37.12 mmHg at the end of one hour of HFNC oxygen therapy (p=0.749). A wide variation was seen in PaO2 levels throughout the study (p=0.458). Baseline pH of 7.35±0.05 improved to 7.40±0.04 after application of HFNC oxygen therapy and acidosis got corrected within 12 hours of HFNC oxygen therapy (p=0.017). This improvement in pH was persistent throughout the end of the study which was statistically significant (p<0.05). The mean hours on HFNC oxygen therapy was 43.27±16.31 hours. One child witnessed failure of HFNC at hour 24 and required invasive ventilation. The adverse events like nasal crusting, epistaxis and pneumothorax were not observed over two months of follow-up.
Baseline parameters on admission.
| Parameters | Result |
|---|
| Mean Heart Rate | 158.50±35.19/min |
| Mean Respiratory Rate | 68.64±10.72/min |
| Mean SpO2 | 88.68±2.12% |
| Mean PaCO2 | 31.23±6.12 mmHg |
| Mean PaO2 | 122.73±44.94 mmHg |
Respiratory rate trends.
p<0.05
Discussion
The study evaluated efficacy and safety parameters of HFNC oxygen therapy in 22 previously healthy children between the ages of 2 months to 2 years diagnosed with moderate and severe bronchiolitis. The mean age (±SD) of children in the study was 7.18±4.48 months which is higher than the recently reported Indian study (5.54±2.11 months) [13]. In this study, there was statistically significant improvement (p<0.05) in the work of breathing as indicated by fall in mean HR and RR along with increase in mean SpO2 levels after 1 hour of HFNC oxygen therapy. This improvement was consistently seen till the end of the study. Similarly, McKiernan C et al., observed a decrease in RR 1-hour after initiation of HFNC oxygen therapy (18±16 breaths/min) compared with (6±14 breaths/min) those who did not receive HFNC oxygen therapy (p<0.001) in infants with bronchiolitis [14]. Improvement in SpO2 levels and decrease in RR and PCO2 was seen with the use of HFNC in infants with moderate to severe bronchiolitis [15].
In this study, significant improvement in the work of breathing (HR, RR and SpO2) did not reflect in the PaO2 parameters. This finding is similar to Oto A et al., where significant improvement in work of breathing was not reflected in ABG parameters (pH, PaCO2, PaO2) even after 12 hours of HFNC oxygen therapy [16]. This needs further research. However, it is to be noted that in this study, there was an improvement in baseline pH 7.35±0.05 to 7.40±0.04 after application of HFNC oxygen therapy and acidosis got corrected within 12 hours of HFNC oxygen therapy (p=0.017). In this study, the mean hours on HFNC oxygen therapy was 43.27±16.31 hours which is higher than reported in an Indian study (33.84±10.8 hours) [13]. Only one child in this study witnessed failure of HFNC at hour 24 and required invasive ventilation. This is similar to data published [13,17] where none of the children, who received HFNC oxygen therapy required invasive ventilation whereas 4 out of 50 children, who received conventional oxygen later developed respiratory failure and needed mechanical ventilation (p<0.04).
In present study, HFNC oxygen therapy was well tolerated by children which is similar to the findings of Mayfield S et al., [18]. However, HFNC is still able to increase end-expiratory pressure despite being an open ventilation system. This is reflected in Hegde S et al., study which reported 3 cases of serious air leaks related to HFNC therapy [19]. Hence, HFNC oxygen therapy requires constant monitoring especially when used outside the PICU setting. A recent retrospective study concluded that the use of HFNC oxygen therapy is safe and efficacious in children aged between 1-23 months with suspected bronchiolitis even in a non-tertiary set-up or ward, with adequate moinitoring and robust transfer criteria [20]. However, in the recently published HFWHO Australia study which was an open, phase 4, randomised controlled trial in children less than 24 months with moderate bronchiolitis, the authors concluded that HFNC usage was not associated with any difference in the time to weaning off oxygen, or the length of stay compared to standard oxygen therapy. However, in children who were non-responders to standard oxygen therapy, HFNC could be looked upon as a rescue option to avoid escalation to invasive respiratory support especially in ICU settings [21]. HFNC was perceived as easy to administer and comfortable for children as per a survey conducted at non-tertiary centres in Australia and New Zealand [22]. This could explain the growing use of HFNC in recent times. Present study has generated positive clinical data on HFNC oxygen therapy in moderate and severe bronchiolitis.
Limitation
Absence of comparative control groups and subgroup analysis are the limitations of the study. Further case-control studies are needed to help establish standardised guidelines regarding using and monitoring HFNC therapy particularly when used outside PICU settings.
Conclusion
HFNC oxygen therapy in moderate and severe bronchiolitis significantly decreases the work of breathing and need of invasive ventilation, and improves oxygen saturation. One patient failed HFNC oxygen therapy and required invasive ventilation. In addition, it was not associated with any known adverse events.
Contributions: All the authors were involved in concept and conduct of the study. SVK analysed and drafted the manuscript. JSO and BS critically reviewed and finalised the manuscript.
[1]. Panitch HB, Callahan JC, Schidlow DV, Bronchiolitis in childrenClinics in Chest Medicine 1993 14(4):715-31. [Google Scholar]
[2]. Shi T, McAllister DA, O’Brien KL, Simoes EA, Madhi SA, Gessner BD, Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling studyThe Lancet 2017 390(10098):946-58.10.1016/S0140-6736(17)30938-8 [Google Scholar] [CrossRef]
[3]. Henderson FW, Clyde Jr WA, Collier AM, Denny FW, Senior RJ, Sheaffer CI, The etiologic and epidemiologic spectrum of bronchiolitis in pediatric practiceThe Journal of Pediatrics 1979 95(2):35-39.10.1016/S0022-3476(79)80647-2 [Google Scholar] [CrossRef]
[4]. Wright RB, Pomerantz WJ, Luria JW, New approaches to respiratory infections in children. Bronchiolitis and croupEmergency medicine clinics of North America 2002 20(1):93-114.10.1016/S0733-8627(03)00053-1 [Google Scholar] [CrossRef]
[5]. Oakley E, Borland M, Neutze J, Acworth J, Krieser D, Dalziel S, Nasogastric hydration versus intravenous hydration for infants with bronchiolitis: a randomised trialThe Lancet Respiratory Medicine 2013 1(2):113-20.10.1016/S2213-2600(12)70053-X [Google Scholar] [CrossRef]
[6]. Sitthikarnkha P, Samransamruajkit R, Prapphal N, Deerojanawong J, Sritippayawan S, High-flow nasal cannula versus conventional oxygen therapy in children with respiratory distressIndian Journal of Critical Care Medicine 2018 22(5):32110.4103/ijccm.IJCCM_181_1729910540 [Google Scholar] [CrossRef] [PubMed]
[7]. Saslow JG, Aghai ZH, Nakhla TA, Hart JJ, Lawrysh R, Stahl GE, Work of breathing using high-flow nasal cannula in preterm infantsJournal of Perinatology 2006 26(8):47610.1038/sj.jp.721153016688202 [Google Scholar] [CrossRef] [PubMed]
[8]. Dani C, Pratesi S, Migliori C, Bertini G, High flow nasal cannula therapy as respiratory support in the preterm infantPediatric pulmonology 2009 44(7):629-34.10.1002/ppul.2105119499590 [Google Scholar] [CrossRef] [PubMed]
[9]. Lee JH, Rehder KJ, Williford L, Cheifetz IM, Turner DA, Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literatureIntensive Care Medicine 2013 39(2):247-57.10.1007/s00134-012-2743-523143331 [Google Scholar] [CrossRef] [PubMed]
[10]. Mayfield S, Jauncey-Cooke J, Hough JL, Schibler A, Bogossian F, High flow nasal cannula therapy for respiratory support in childrenCochrane Database of Systematic Reviews 2014 (3):CD009850-pub2.10.1002/14651858.CD009850.pub224604698 [Google Scholar] [CrossRef] [PubMed]
[11]. Nishimura M, High-flow nasal cannula oxygen therapy in adultsJournal of Intensive Care 2015 3(1):1510.1186/s40560-015-0084-525866645 [Google Scholar] [CrossRef] [PubMed]
[12]. Mikalsen IB, Davis P, Øymar K, High flow nasal cannula in children: a literature reviewScandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2016 24(1):9310.1186/s13049-016-0278-427405336 [Google Scholar] [CrossRef] [PubMed]
[13]. Ahmed P, Maqbool J, Ashraf M, Humidified high flow nasal cannula oxygen therapy in acute bronchiolitisIndian Journal of Child Health 2017 4(2):133-35. [Google Scholar]
[14]. McKiernan C, Chua LC, Visintainer PF, Allen H, High flow nasal cannulae therapy in infants with bronchiolitisThe Journal of Pediatrics 2010 156(4):634-38.10.1016/j.jpeds.2009.10.03920036376 [Google Scholar] [CrossRef] [PubMed]
[15]. Bressan S, Balzani M, Krauss B, Pettenazzo A, Zanconato S, Baraldi E, High-flow nasal cannula oxygen for bronchiolitis in a pediatric ward: a pilot studyEuropean Journal of Pediatrics 2013 172(12):1649-56.10.1007/s00431-013-2094-423900520 [Google Scholar] [CrossRef] [PubMed]
[16]. Oto A, Erdogan S, Bosnak M, Oxygen therapy via high flow nasal cannula in pediatric intensive care unitThe Turkish Journal of Pediatrics 2016 58(4):37710.24953/turkjped.2016.04.00528276209 [Google Scholar] [CrossRef] [PubMed]
[17]. Schibler A, Pham TM, Dunster KR, Foster K, Barlow A, Gibbons K, Hough JL, Reduced intubation rates for infants after introduction of high-flow nasal prong oxygen deliveryIntensive Care Medicine 2011 37(5):847-52.10.1007/s00134-011-2177-521369809 [Google Scholar] [CrossRef] [PubMed]
[18]. Mayfield S, Bogossian F, O’Malley L, Schibler A, High-flow nasal cannula oxygen therapy for infants with bronchiolitis: pilot studyJournal of Paediatrics and Child Health 2014 50(5):373-78.10.1111/jpc.1250924612137 [Google Scholar] [CrossRef] [PubMed]
[19]. Hegde S, Prodhan P, Serious air leak syndrome complicating high-flow nasal cannula therapy: a report of 3 casesPediatrics 2013 :peds-2011.10.1542/peds.2011-376723382446 [Google Scholar] [CrossRef] [PubMed]
[20]. Davison M, Watson M, Wockner L, Kinnear F, Paediatric high-flow nasal cannula therapy in children with bronchiolitis: A retrospective safety and efficacy study in a non-tertiary environmentEmergency Medicine Australasia 2017 29(2):198-203.10.1111/1742-6723.1274128332328 [Google Scholar] [CrossRef] [PubMed]
[21]. Kepreotes E, Whitehead B, Attia J, Oldmeadow C, Collison A, Searles A, High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trialThe Lancet 2017 389(10072):930-39.10.1016/S0140-6736(17)30061-2 [Google Scholar] [CrossRef]
[22]. Manley BJ, Owen L, Doyle LW, Davis PG, High-flow nasal cannulae and nasal continuous positive airway pressure use in non-tertiary special care nurseries in Australia and New ZealandJournal of Paediatrics and Child Health 2012 48(1):16-21.10.1111/j.1440-1754.2011.02186.x21988616 [Google Scholar] [CrossRef] [PubMed]