Fungal keratitis is a common cause of corneal blindness in the developing world. The incidence is far more common in the Indian subcontinent, where it has been associated with almost half of all corneal ulcers [1,2]. The outcomes of keratomycosis using conventional treatment regimens (which include use of topical and oral antifungal agents) are far from optimal [3-5]. This may be due to several short-comings of commonly used antifungal agents, including poor ocular penetration, fungistatic nature, drug stability, lack of broad-spectrum coverage, and suboptimal aqueous concentration [6].
A few investigators have reported success using intracameral delivery of antifungal agents, including Amphotericin B [4,7]. Amphotericin B is a macrocyclic polyene and is effective against a broad spectrum of fungi, both filamentous and yeast forms [8-11]. Significant drug concentration can be achieved in the anterior chamber following intracameral injection of Amphotericin B [12]. In addition, it has been reported to be safe and well tolerated. A limited clinical data regarding efficacy of ICAMB suggests that it may have an important role in advanced or non-responding cases of fungal keratitis. Early intervention with ICAMB may decrease, both the duration of disease as well as the ocular morbidity associated with severe fungal corneal ulcers.
Authors, therefore, decided to study the treatment outcomes, efficacy and safety of early versus delayed intervention using intracameral injection of Amphotericin B in severe, non-responding keratomycosis.
Materials and Methods
A total of 50 eyes of 50 patients with microbiologically proven ‘severe’ fungal keratitis presenting to the Cornea Services, Advanced Eye Centre, Post Graduate Institute of Medical Education and Research, Chandigarh, India were enrolled from January 2006 to January 2009 for the study. This prospective, interventional study was approved by the Ethics Committee and adhered to the tenets of Declaration of Helsinki.
Severe corneal ulcer was defined as ulcer with infiltrates more than 5 mm in size and involving more than 2/3rd of the corneal thickness [13]. All eyes had microbiologically proven fungal corneal ulcer with either 10% KOH wet mount/Calcofluor white stain (CFW) smear positivity or growth of fungi on Sabouraud’s Dextrose Agar (SDA). All patients were above 18 years of age and were willing to be treated as an in-patient or as an outpatient and to follow-up every 48-72 hours for the appropriate intervention. Patients with a perforated corneal ulcer, impending corneal perforation, scleral involvement, endophthalmitis, polymicrobial infection, known allergy to antifungals, systemically immune-compromised status, hepatic or renal disease or deranged Liver Function Tests (LFT) and those not willing to participate were excluded from the study. A detailed written informed consent was taken from every patient.
After obtaining demographic data and history regarding the events leading to the ulcer, a complete ocular examination was done. This included a detailed slit lamp examination of the cornea including colour-coded diagrams, fluoresce in staining of cornea and digital tonometry. The Best-Corrected Visual Acuity (BCVA) was recorded in all cases and posterior segment was evaluated either by indirect ophthalmoscopy wherever fundus was visualised or by ultrasound if the infiltrates were blocking the view of fundus. The size of the epithelial defect, depth and size of the infiltrate and presence of hypopyon were evaluated at presentation and subsequently at each visit. The ulcers were graded as mild, moderate or severe using Jones criteria [14].
Corneal scraping was carried out using a sterile Bard–Parker blade no. 15 under topical 4% lidocaine/0.5% proparacaine anaesthesia. Corneal scraping was done under direct visualisation through the slit lamp. The scrapings were obtained from multiple sites at the advancing edge and base of the ulcer and were sent for direct microscopy and culture. For direct microscopic examination, 10% potassium hydroxide/calcofluor white stained slides were used and were examined under ultraviolet microscope. Additionally, gram stained slides were examined under light microscope for bacteria. The remaining material was inoculated onto sheep blood agar, chocolate agar, SDA with chloramphenicol and brain heart infusion agar. A pattern of ‘C’ shaped inoculation on SDA plate was done. SDA plates for fungal culture were incubated at 25°C and were examined daily for first week and twice daily thereafter for four weeks. Bacterial cultures were incubated at 37°C and were evaluated at 24 and 48-hours of incubation. The fungal aetiology was confirmed when fungal profile was observed on direct microscopy, with or without the growth of fungi along the ‘C’ pattern of inoculation.
In all patients, conventional antifungal therapy was initiated which included administration of 5% topical natamycin suspension every one hourly. In addition, prophylactic antibiotic therapy in the form of moxifloxacin 0.5% along with atropine 1% three times a day was instituted. Oral itraconazole 200 mg BD was given in all cases. Initially, the above treatment regimen was followed in all patients for a period of two weeks.
The patients were assessed every 48-hours to note the change in the characteristics of the ulcer and response to treatment. A favourable response included the rounding of infiltrate margin, decrease in the size of epithelial defect or endothelial plaque. Patients who did not improve after two weeks of intensive antifungal therapy were randomly allocated to Group A or to Group B using random number tables. Group A received ICAMB at two weeks while Group B underwent the same procedure at four weeks (while intensive antifungal therapy was continued). All patients continued to receive the standard antifungal therapy along with the above intervention.
Preparation and Procedure for Intracameral Amphotericin B Injection
Intracameral Amphotericin B was prepared by diluting 50 mg of liposomal Amphotericin B injection vial (Fungizone, Sarabhai Chemicals, Vadodara, India) with 5 mL of sterile water for injection under laminar flow hood. 1 mL (10 mg) of this solution was again diluted with 9 mL of sterile water to get a concentration of 1 mg/mL. Out of this, 9 mL was discarded and 9 mL of sterile water was again added to get a concentration of 0.1 mg/mL which contained 10 μg of Amphotericin B per 0.1 mL.
After taking informed consent, the procedure was carried out under an operating microscope using strict aseptic precautions. Topical proparacaine 0.5% was instilled in the conjunctival sac 2 minutes before the injection. In patients who were unwilling for carrying out the procedure under topical anaesthesia patients, a peribulbar block was given using a combination of 5 mL bupivacaine 0.5% and 5 mL of lidocaine 2% with 1:200000 unit’s adrenaline and 150 unit’s of hyaluronidase. Paracentesis of approximately 0.1 mL of aqueous was done with a 26G needle attached to a 2 mL syringe without the piston. The site of paracentesis was either the temporal or the superior limbus in an area free of infiltrates. Thereafter, a preloaded tuberculin syringe was used to inject 0.1 mL of Amphotericin B into the anterior chamber through the same site as the paracentesis. The standard antifungal topical and systemic therapy was continued as before.
In patients failing to show a response on clinical examination, repeat injections were administered every 48-72 hours up to a maximum cumulative dose of 50 μg of ICAMB. The endpoint was either a resolution of infiltrates or a maximum of five injections. All patients were followed for a period of three months.
Treatment success was defined as resolution of the corneal infiltrate with scarring, disappearance of the corneal endothelial plaque and hypopyon, and healing of the epithelial defect. Treatment failure was considered if: i) the infiltrate and/or epithelial defect increased in size; or ii) increase in the size of hypopyon or endothelial plaque; or iii) if there was a corneal perforation. In addition, we studied the anatomical and functional outcome in these eyes as follows:
a) Anatomical outcome- i.e., the type of corneal opacity (maculo-leucomatous corneal opacity, leucomatous corneal opacity or adherent leucoma).
b) Functional outcome- the final visual acuity.
Any complications including cataract, corneal perforation, hyphema as well as any surgical intervention performed including therapeutic keratoplasty, anterior chamber wash and glue application etc., were noted in all eyes.
Statistical Analysis
Descriptive statistics were used to estimate mean values with their 95% confidence intervals for quantitative variables. Qualitative data were expressed as proportion. Q-Q plots and Kolmogorov Smirnov tests assessed normality of data. In view of small sample size and non-normality of data, non-parametric statistics were used for this study. Qualitative variables were assessed between two groups of intervention using chi-square test and Fisher-exact test where applicable. Quantitative patient characteristics were compared between the two groups of intervention using Mann-Whitney U test. Baseline and final visual acuity in each group were compared using Wilcoxon signed rank test to account for dependent observations. Outcome measure of time to heal was analysed between the two treatment groups using survival analysis with Kaplan Meier curves. All tests were two-tailed and p-value <0.05 was taken as significant. A Spearman’s Correlation of the healing time with other clinical characteristics was also done. All the calculation was done using SPSS software version 25.0.
Results
The present study included 50 eyes of 50 patients with proven fungal corneal ulcer presenting to the cornea clinic, Advanced Eye Centre, Post Graduation Institute of Medical Education and Research, Chandigarh. Group A comprised of patients who received ICAMB at the end of two weeks (early intervention group) and Group B of patients who received ICAMB at four weeks (delayed intervention group) following conventional antifungal therapy.
The mean age of patients in Group A was 38.4±11.2 years and in Group B 38.8±12.6 years. 22 (88%) patients in Group A and 21 (84%) in Group B were males. All patients in both the groups had unilateral involvement. The mean duration of signs and symptoms pertaining to the corneal ulcer was 32.2 days in Group A and 28.7 days in Group B at the time of presentation. There was no statistically significant difference in the demographic profile of patients in the two groups (p>0.05).
The visual acuity at presentation in the early, as well as delayed intervention groups, was <6/60 in all eyes. In Group A, 2 (8%) eyes had an initial visual acuity between 6/60 to 3/60, 12 (48%) eyes were 3/60 to CFCF (counting fingers close to face) while 11 (44%) eyes were hand motion or worse, while in Group B, 7 (28%) eyes had an initial visual acuity between 3/60 to CFCF and 18 (72%) eyes were hand motion or worse. The clinical characteristics of the ulcers are given in [Table/Fig-1]. There was no statistically significant difference in the morphological characteristics between the two groups except for corneal thinning, which was present in 6 (24%) eyes in Group A versus 13 (52%) eyes in Group B (p=0.04). Authors included corneal ulcers which were positive for the presence of fungal elements on corneal smear examination (10% KOH wet mount/Calcofluor white stain). In addition, fungal cultures were positive in 18 (36%) eyes. The distribution of fungal culture isolates between the two groups is given in [Table/Fig-2].
Morphological features of fungal corneal ulcers in Group A versus Group B.
| Variables | Group A [no of patients (%)] | Group B [no. of patients (%)] | Total (number of patients=50) |
|---|
| Mean infiltrate size (mm) | 6.05±1.31 | 6.37±1.08 | 6.22±1.19 |
| Full thickness infiltrates | 18 (72) | 23 (92) | 41 (82) |
| Satellite lesions | 6 (24) | 22 (88) | 28 (56) |
| Hypopyon | 22 (88) | 24 (96) | 46 (92) |
| Endothelial plaque | 14 (56) | 15 (60) | 29 (58) |
| Corneal thinning | 6 (24) | 13 (52) | 19 (38) |
| AC Abscess | 9 (36) | 15 (60) | 24 (48) |
Comparison of fungal culture isolates in the study population.
| Organism | No of eyes (%) (n=50) | Group A (n=25) (%) | Group B (n=25) (%) |
|---|
| Aspergillus Flavus | 9 (18) | 5 (20) | 4 (16) |
| Fusarium solaris | 4 (8) | 1 (4) | 3 (12) |
| Aspergillus niger | 2 (4) | 2 (8) | 0 |
| Curvularia lunata | 2 (4) | 0 | 2 (8) |
| Bipolaris spicifera | 1 (2) | 0 | 1 (4) |
| No organism cultured* | 32 (64) | 17 (68) | 15 (60) |
*These ulcers were smear positive for fungi but culture negative
A mean of 4 (range 3-5) injections of intracameral Amphotericin B was received by patients in Group A and five injections in Group B (p=0.008). Of the 25 eyes in Group A, 7 (28%) eyes required three injections, 9 (36%) eyes required four injections and only 9 (36%) required five injections for the ulcer to heal, while in Group B only 3 (12%) eyes responded to three injections, 3 (12%) eyes to four injections and 19 (76%) eyes required five injections. The mean time for healing of the fungal ulcer was 17.5±3.64 days in Group A and 32.2±8.89 days in Group B (p<0.001).
The anatomical outcome in the form of the nature of corneal opacity and vascularisation between the two groups is illustrated in [Table/Fig-3a]. The functional outcome in the form of final visual acuity is illustrated in [Table/Fig-3b]. Successful outcomes after early intervention with ICAMB can be seen in [Table/Fig-4].
| Groups | Maculo-leucomatous | Leucomatous | Adherent leucoma | Vascularised corneal opacity |
|---|
| Group A | 12 (48%) | 13 (52%) | 0 | 9 (36%) |
| Group B | 4 (16%) | 16 (64%) | 5 (20%) | 17 (68%) |
| 6/12-6/36 | 6/60 | 6/60-3/60 | 3/60-CFCF | HM |
|---|
| Final visual acuity | Group A | 2 (8) | 1 (4) | 4 (16) | 15 (60) | 3 (12) |
| Group B | 0 | 0 | 4 (16) | 12 (48) | 9 (36) |
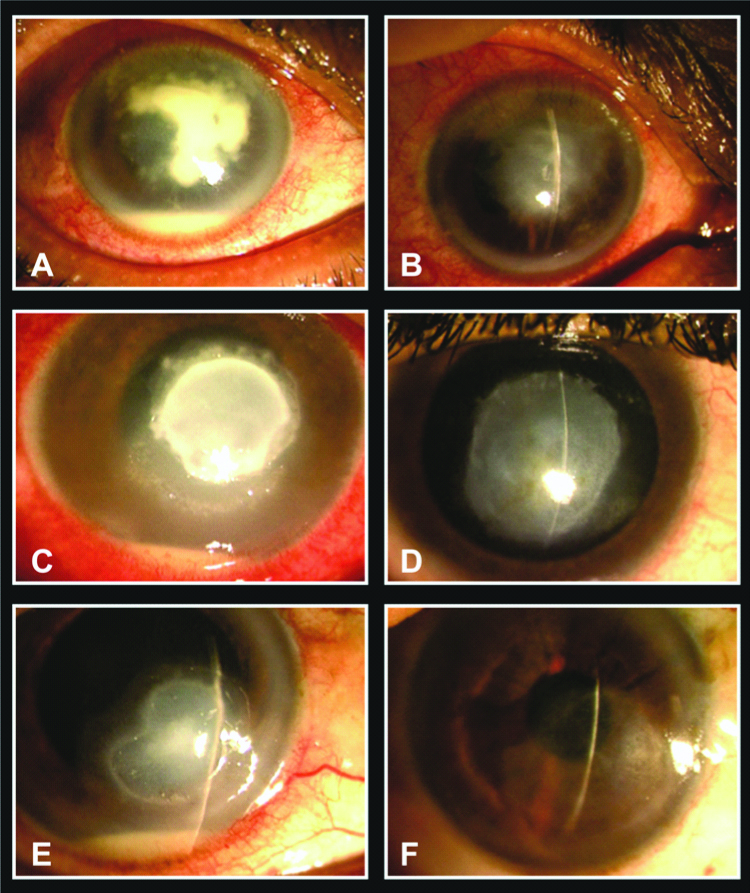
Successful outcomes following ‘early’ intervention with ICAMB. A) Non-responding corneal ulcer with hypopyon, endothelial plaque and anterior chamber abscess; B) After 5 injections of ICAMB, the infiltrates and abscess resolved leaving a non-vascularised corneal opacity; C) Severe fungal corneal ulcer with thick, dry, raised infiltrates and satellite lesions; D) Complete resolution following intervention using ICAMB; E) A resolving fungal corneal ulcer following 2 intracameral injections of Amphotericin B; F) The ulcer resolved with minimal scarring leaving a maculo-leucomatous opacity. Note the complete lack of vascularisation.
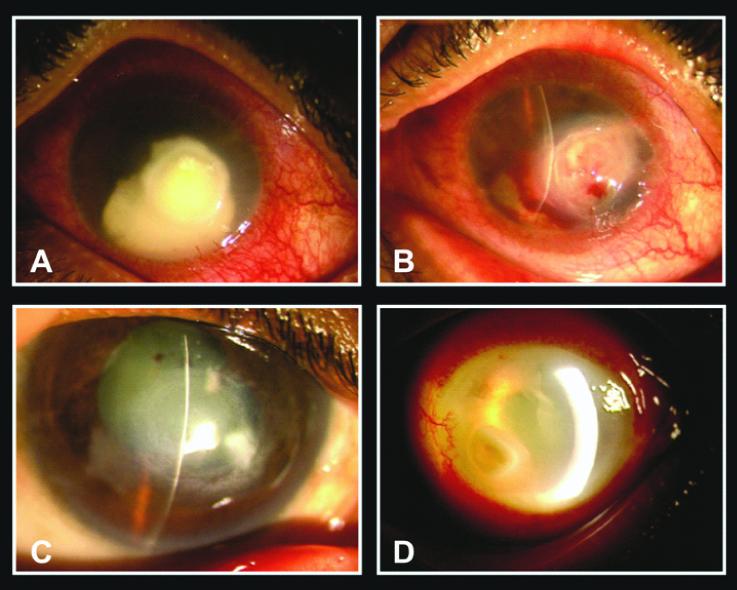
Seven (28%) eyes in Group A and 16 (64%) eyes in Group B had complications following intracameral injections of liposomal Amphotericin B. 6 (24%) eyes in Group A and 14 (56%) eyes in Group B developed a cataract (p=0.04). 1 (4%) eyes in Group A and 2 (8%) in Group B developed hyphema following ICAMB (p=0.05). An increase in the anterior chamber inflammation was noted in 3 (12%) eyes in Group A and 4 (16%) eyes in Group B respectively. Eight percent of patients complained of significant pain lasting for 2-3 hours in both the groups. In addition, 10 (40%) eyes of Group B developed a corneal perforation and ultimately underwent a penetrating keratoplasty while none in Group A had a corneal perforation (p=0.006). An additional surgical intervention in the form of an anterior chamber wash was required in 3 (12%) eyes in Group A and 4 (16%) eyes in Group B. Anterior segment images showing various complications after ICAMB [Table/Fig-5].
Complications of ICAMB. A) Corneal ulcer with anterior chamber abscess before initiating ICAMB; B) Hyphema and intrastromal haemorrhage following Intracameral injection of Amphotericin B; C) Maculo-leucomatous opacity and complicated cataract following resolution of ulcer after ICAMB therapy; D) Increased anterior chamber inflammation and thick, fibrinous exudates 1 day after ICAMB.
Mann-Whitney U-test was done for comparative analysis of quantitative variables (age, duration of illness, infiltrate size, healing time and number of injections) between the two intervention groups. Early intervention with ICAMB was found to be significantly associated with lesser time required for healing of the fungal corneal ulcer (p<0.001) [Table/Fig-6]. Spearman’s Correlation of the healing time with other clinical characteristics was also done [Table/Fig-7]. A significant positive correlation was noted between the healing time and the duration of illness (r=0.29, p-value=0.04), worse initial visual acuity (r=0.75, p<0.001), number of injections (r=0.29, p=0.03), and final visual acuity (r=0.33, p=0.01). This reflects significantly longer healing time in patients with longer duration of illness, worse initial visual acuity and in those who received higher number of injections. Kaplan Meier curves showed that the healing time was significantly shorter in early intervention as compared to late intervention. The log rank test between two interventions was 23.43 (p-value <0.001) [Table/Fig-8].
Comparative analysis of quantitative variables between two intervention groups.
| Variables | Type of intervention | Mann-Whitney U-test (p-value) |
|---|
| Early | Delayed |
|---|
| Mean (95% CI) | Mean (95%) |
|---|
| Age (years) | 38.48 (33.81-43.14) | 38.84 (33.57-44.10) | 0.96 |
| Duration of illness (days) | 32.2 (28.14-36.19) | 43.12 (37.31-48.86) | 0.004 |
| Infiltrate size | 6.04 (5.50-6.58) | 6.37 (5.92-6.82) | 0.27 |
| Healing time (days) | 18.06 (16.45-19.6) | 36.68 (34.93-38.36) | <0.001 |
Spearman correlation of healing time with other clinical characteristics.
| Healing time (wks) | Age | Duration of illness (wks) | Initial VA | Infiltrate size | No. of injections | Final VA |
|---|
| Healing time (wks) | 1.000 | | | | | | |
| Age | 0.005 | 1.000 | | | | | |
| 0.970 | . | | | | | |
| Duration of illness (wks) | 0.290* | -0.049 | 1.000 | | | | |
| 0.041 | 0.734 | . | | | | |
| Initial VA | 0.756** | -0.030 | 0.416** | 1.000 | | | |
| <.0001 | 0.837 | 0.003 | . | | | |
| Infiltrate size | 0.248 | 0.011 | 0.429** | 0.168 | 1.000 | | |
| 0.082 | 0.938 | 0.002 | 0.243 | . | | |
| No. of injections | 0.298* | 0.055 | 0.444** | 0.382** | 0.116 | 1.000 | |
| 0.035 | 0.704 | 0.001 | 0.006 | 0.424 | . | |
| Final VA | 0.339* | -0.018 | 0.162 | 0.453** | 0.205 | 0.254 | 1.000 |
| 0.016 | 0.903 | 0.262 | 0.001 | 0.152 | 0.075 | . |
*Correlation is significant at the .05 level (2-tailed);
**Correlation is significant at the .01 level (2-tailed)
The comparison of healing time in early versus late intervention using log rank test.
| Intervention group | Sample (n) | Censored* n (%) | Events# | Mean (median) time to heal wks) | 95% CI | Log rank test | p-value |
|---|
| Early | 25 | 7 (28%) | 18 | 2.83 (3) | 2.55-3.11 | 23.43 | <0.001 |
| Late | 25 | 2 (8%) | 23 | 4.74 (5) | 4.22-5.25 | | |
*Censoring was done for superficial corneal ulcers;
#Events were patients having only deep corneal ulcer
Discussion
In the present study, the authors intervened using ICAMB after failure of two weeks of intensive antifungal treatment. At the same time, a delayed intervention group (four weeks) was also studied, in keeping with the tendency on part of the treating ophthalmologists of delaying intervention using this modality [15,16]. Several authors have reported on the efficacy of use of intracameral Amphotericin B in fungal corneal ulcers. The dose of intracameral Amphotericin B varied from 5-10 mg per injection and mean number of injections varied from 2-13 in these studies. The average time of intervention with intracameral Amphotericin B was 24.36 days (Range 12.45-4) [Table/Fig-9] [7,15-19]. Since none of the studies has compared the efficacy of early versus delayed intervention, hence authors designed this study. An early intervention offered several advantages in managing non-responding keratomycosis including significantly less number of injections (mean 4 versus 5 per eye), significantly reduced healing time 17.5±3.64 days versus as 32.2±8.89 days. (p<0.001). In addition, A Spearmann correlation test revealed that faster healing of the corneal ulcer was positively correlated with duration of illness, initial visual acuity, number of injections and final visual acuity (In other words, patients with longer duration of illness, worse initial visual acuity and those who received higher number of injections took longer to heal).
A comparison of published studies showing efficacy of intracameral Amphotericin B with our study.
| Study | Number of eyes | Max no of injections | Concentration of ICAMB (μg) | Timing of intervention (days) | Hypopyon | Healing time (days) | Disappearance of hypopyon (days) |
|---|
| Kaushik et al., [15] 2001 | 3 | 2 | 7.5-10 | 40 | present | 21 | 21 |
| Kuriakose et al., [16] 2002 | 4 | 13 | 5 | 28 | present | 14 | 12 |
| Yilmaz et al., [18] 2005 | 14 | 5 | 5 | 17±4.77 | present | 32±11.67 | 6.07±2.07 |
| Yoon et al., [7] 2007 | 14 | 5 | 10 | NM | present | 19.8±10.4 | 9.4±9.4 |
| Shao et al., [19] 2010 | 30 | 2 | 10 | NM | present | 20.2±10.6 | 9.6±9.2 |
| Sharma et al., [17] 2015 | 52 | NM | 5-10 | 12.45±9.08 | present | 12.37±5.5 | 13.4±8 |
| Early intervention (present study) | 25 | 5 | 10 | 14 | present | 18.06 (16.45-19.6) | |
| Delayed Intervention (present study) | 25 | 5 | 10 | 28 | present | 36.68 (34.93-38.36) | |
Various studies have shown that patients receiving intracameral Amphotericin B have a corneal perforation rate varying between 6-25% [15-17]. In the present study, authors observed a higher rate of corneal perforation and need for therapeutic penetrating keratoplasty {10 (40%) eyes versus none in delayed versus early intervention groups}. This was the most significant impact that authors noted with an early intervention using ICAMB. It is possible that the worse outcome noted in terms of healing time, corneal perforation etc. may be due to more advanced disease in the delayed group rather than early ICAMB intervention.
Limitation
One of the limitations of this study appears to be the fact that worse outcomes in the ‘delayed’ intervention could be simply attributed to intervention using ICAMB at four weeks instead of two weeks in a non-responding fungal corneal ulcer. While this is possible, a comparison of the morphological characteristics of the ulcers at the time of inclusion in both the groups was not as varied to warrant the difference in outcomes between the two groups. A larger prospective clinical trial may answer this question.
Conclusion
The present data suggest that early intervention using intracameral injection of Amphotericin B may be a viable and effective treatment option in patients suffering from severe fungal corneal ulcers, which fail to respond to ‘conventional’ antifungal therapy. Most significantly, it appears that early intervention using ICAMB reduces the need for performing therapeutic corneal transplants, thus having an impact on reducing ocular blindness and morbidity.
*These ulcers were smear positive for fungi but culture negative
*Correlation is significant at the .05 level (2-tailed);
**Correlation is significant at the .01 level (2-tailed)
*Censoring was done for superficial corneal ulcers;
#Events were patients having only deep corneal ulcer