Role of Serum Vitamin D Level in Progression of Diabetic Foot Ulcer
Farzad Najafipour1, Naser Aghamohammadza2, Neda Razzaghi Zonouz3, Jalil Houshyar4
1 Endocrine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
2 Endocrine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
3 Department of Dermatology, Sina Hospital, Tabriz University of Medical Sciences, Tabriz, Iran.
4 Endocrine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Jalil Houshyar, Endocrine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
E-mail: Jalilhoushyar@yahoo.com
Introduction
Diabetes mellitus (DM) is a metabolic disorder characterised by hyperglycemia. Deficiency of vitamin D has been shown to interfere with insulin production and secretion and therefore contribute to type 2 diabetes development. It has also been noted that vitamin D plays a role in diabetic neuropathy development, which might lead to diabetic foot ulcers (DFU). Vitamin D boosts the immune system, helps in the elimination of bacteria and thus accelerates wound healing and prevents ulcer formation. However, different studies report conflicting results on the association of level of vitamin D with diabetic foot ulcers in DM patients.
Aim
To evaluate the role of serum vitamin D levels with diabetic foot ulcer formation and progression.
Materials and Methods
In this study, 70 diabetic patients with or without DFU either in inpatient section or referring to Imam Reza Hospital Endocrinology Clinic of Tabriz University of Medical Sciences were enrolled. Apparently healthy (n=35) individuals were recruited as the control group. The general characteristics of patients were assessed. The foot ulcers were examined and classified as per Wagner ulcer classification system. Serum biochemical markers including 25-Hydroxy Vitamin D (25(OH)D) were analysed using in-vitro chemiluminescent immunoassay (CLIA). ANOVA and post-hoc (Tukey) tests were employed to compare the means among the groups. Multiple linear regression and bivariate correlation analysis were also conducted to assess the association between all clinical variables and 25(OH)D. The p-value <0.05 was considered as statistically significant.
Results
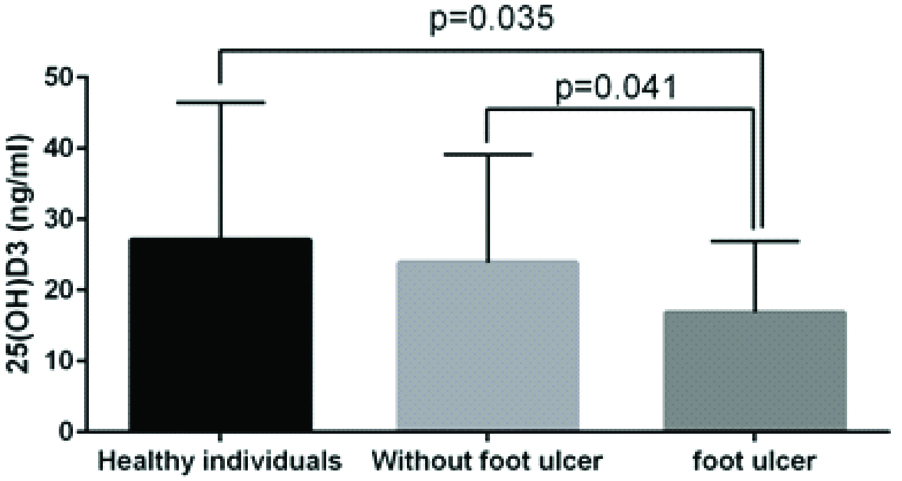
Serum 25(OH)D levels in diabetic patients with or without foot ulcers and healthy controls were 16.86±10, 23.9±15.24 and 27.11±19.35 ng/mL, respectively. Significant differences in 25(OH)D levels were observed between patients and healthy controls (p=0.035), and between diabetic patients with and without foot ulcers (p=0.029). Furthermore, a negative correlation between serum cholesterol and 25(OH)D levels were observed among patient without DFU (-0.401, p-value =0.017).
Conclusion
Low levels of vitamin D in diabetic patients may be related to DFU formation and development. Therefore, early estimation of 25(OH)D and prescription of appropriate vitamin D supplements are suggested in diabetic patients.
Biochemical markers, Diabetes mellitus, Hyperglycaemia
Introduction
Characterised by hyperglycaemia, diabetes mellitus is a metabolic disorder, aetiologically categorised into four types, based on the destruction of pancreas insulin-producing cells, progressive impairment of insulin secretion due to insulin resistance, gestational diabetes and diabetes with other aetiologies [1]. It is estimated that there will be 642 million diabetic patients by 2045 [2]. Additionally, a recent prospective study has estimated the proportions of Type 1, Type 2 and the other types of diabetes as 11.7%, 85.5% and 1.3%, respectively in Iran [3]. Diabetes mellitus is associated with various clinical complications, one of the most serious of which is foot ulcer. About 10-25% of diabetic patients suffer from foot ulcer, which often leads to amputation in severe cases. As a result, diabetes mellitus has been accepted as the most common cause of non-traumatic amputation worldwide [4].
Vitamin D deficiency has been considered as an increasing global concern threatening public health. The main biological role of vitamin D is calcium homeostasis and bone formation. However, its functions and association with diabetic disorders and cardiovascular diseases have also been studied [5]. Vitamin D also plays a crucial role in the regulation of the anti-microbial activity via the expression of Vitamin D Receptor (VDR) in a variety of immune system cells including neutrophils, macrophages, TCD4+, TCD8+ and B-cells. Vitamin D reduces the production of pro-inflammatory cytokines, elevates anti-inflammatory responses, participates in wound-healing processes, insulin resistance prevention and induction of VDR expression [3,5]. DFU infection is a major concern which is augmented by the deficiency in vitamin D [6,7]. Given the importance of vitamin D in immune reactions and diabetic complications, the present study aimed to compare serum vitamin D status in diabetic patients with or without foot ulcer and healthy subjects.
Materials and Methods
In the present study, 105 (power of study: 80%; confidence interval: 95%) subjects either in inpatient section or referring to Imam Reza Hospital Endocrinology Clinics of Tabriz University of Medical Sciences, Iran from August 2017 to August 2018 were enrolled. Participants aged 18 to 80 years, were divided into three groups: diabetic patients with (n=35) or without (n=35) foot ulcer and apparently healthy control subjects (n=35).
This study was approved by the Research Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1396.255). Written informed consent was also obtained from all patients prior to any intervention.
The diabetic patients with or without foot ulcer involvement were selected according to the American Diabetes Association (ADA) criteria. Pregnant women or patients with vitamin D supplement consumption over the past six months, patients with cancer, rheumatologic, blood, cardiovascular, lung, and moderate to severe renal or liver diseases were excluded from the study. The general characteristics of patients such as age, sex, diabetes duration, BMI and grade of ulcer were analysed using a questionnaire. The foot ulcer was examined and classified as per Wagner ulcer classification system. Overnight fasting blood (8 mL) was collected and serum biochemical markers and 25(OH)D were estimated using a Hitachi 902 chemistry analyser (Japan) and Liaisonanalyser (Italy) respectively. The samples were analysed on the same day of collection using 25-(OH) total vitamin D assay kits (Liaison, Diasorin,133636, 133713) based on in-vitro Chemiluminescent Immunoassay (CLIA).
Statistical Analysis
Data were presented as Mean±SD or percent and analysed using Statistical Package for Social Sciences (SPSS) version 23.0 (IBM Corporation, USA). ANOVA and post-hoc (Tukey) tests were employed to compare the means among the groups. Multiple linear regression and bivariate correlation analysis were also conducted to assess the association between all clinical variables and 25(OH) D, which independently predicted foot ulcer development. A p-value <0.05 was considered as statistically significant.
Results
The general characteristics of patient and other biochemical parameters of the participants under study were analysed [Table/Fig-1]. Reduced levels of this vitamin were observed in patients with and without DFU as compared to healthy controls (16.86±10, 23.9±15.24 and 27.11±19.35, respectively). However, the differences were significant only between DFU and healthy controls (p=0.035) and DFU and non-DFU patients (p=0.041) [Table/Fig-2]. Multiple linear regression analysis to study the independent variables predicting foot ulcer in diabetic patients and healthy controls showed that low plasma 25(OH)D (ng/mL) was correlated with foot ulcer by linear regression (r: -0.119, p=0.016). In this analysis, the serum 25(OH)D (ng/mL) was considered as an independent variable for the model and only the variables with a p-value <0.05 were considered in the final fitted model.
General characteristics and biochemical parameters of participants under study.
| Factors | Healthy individuals n=35 | Without foot ulcer n=35 | Foot ulcer n=35 | p-value |
|---|
| Sex (male %) | 40 | 48.6 | 77.1 | - |
| Age (year) | 41.77±13.58 | 56.34±14.22a | 62.09±11.23a | <0.001* |
| Diabetes duration (year) | - | 6.69±5.09 | 17.24±9.26b | <0.001* |
| BMI (kg/m2) | 27.07±5.79 | 30.26±5.84a | 26.55±3.21b | 0.006* |
| Grade of ulcer |
| Grade 2 | - | - | 6 | - |
| Grade 3 | - | - | 14 | - |
| Grade 4 | - | - | 15 | - |
| FBS (mg/dL) | 95.6±11.96 | 176.2±90.58a | 175.22±86.06a | <0.001* |
| HbA1C (%) | - | 7.4±0.98 | 9.33±2.54b | <0.001* |
| LDL (mg/dL) | 119±39.9 | 97.95±27.29a | 73.6±27.24a,b | <0.001* |
| HDL (mg/dL) | 44.74±7.9 | 41.14±8.75 | 30.71±14.7a,b | <0.001* |
| TG (mg/dL) | 166.8±109.36 | 189.77±119.37 | 124.68±73.9b | 0.03* |
| Chol (mg/dL) | 191.6±51.06 | 177.28±35.54 | 129.4±38.92a,b | <0.001* |
| Cr (mg/dL) | 0.9±0.16 | 1.06±0.23 | 1.66±1.47a,b | 0.001* |
| Hg (g/dL) | 13.52±1.43 | 13.83±1.5 | 11.1±2.12a,b | <0.001* |
| ESR (mm/h) | 12.47±8.18 | 10.82±8.04 | 74.34±41.59a,b | <0.001* |
| 25 (OH)D (ng/mL) | 27.11±19.35 | 23.9±15.24 | 16.86±10a,b | <0.05* |
Data are presented as mean±SD or percent. ANOVA and post-hoc (Tukey) test were used for analysis. (ap<0.05 versus healthy individuals, bp<0.05 versus without foot ulcer group)
BMI: Body mass index; FBS: Fasting blood sugar; HbA1C: Haemoglubin A1C; LDL: Low density lipoprotein; HDL: High density lipoprotein; TG: Triglyceride; Chol: Cholesterol; Cr: Creatinine; Hg: Haemoglubin; ESR: Erythrocyte sedimentation rate; 25(OH)D: 25-hydroxy vitamin D
Vitamin D levels in type 2 diabetic patients with and without DFUs and healthy controls.
Data were adjusted for age and BMI. Valuesare presented as mean±SD. ANOVA and post-hoc (Tukey) tests were employed to compare the means among the groups. p<0.05 was considered as statistically significant
Correlation analysis between 25(OH)D levels and laboratory and clinical variablesin patients with or without DFU.
| Independent variable | Serum 25 (OH)D (ng/mL) |
|---|
| Without DFU (n=35) | DFU (n=35) |
|---|
| r-value | p-value | r-value | p-value |
|---|
| Age (year) | 0.201 | 0.247 | 0.276 | 0.108 |
| Diabetes duration (year) | 0.314 | 0.066 | -0.141 | 0.421 |
| BMI (kg/m2) | -0.134 | 0.442 | -0.036 | 0.836 |
| Grade of ulcer | - | - | 0.194 | 0.265 |
| HbA1C (%) | 0.077 | 0.687 | -0.189 | 0.277 |
| FBS (mg/dL) | -0.122 | 0.486 | -0.25 | 0.147 |
| LDL (mg/dL) | -0.311 | 0.069 | -0.019 | 0.912 |
| HDL (mg/dL) | -0.169 | 0.332 | -0.065 | 0.710 |
| TG (mg/dL) | -0.177 | 0.31 | -0.195 | 0.261 |
| Chol (mg/dL) | -0.401 | 0.017* | -0.112 | 0.522 |
| Cr (mg/dL) | 0.01 | 0.952 | 0.243 | 0.160 |
| Hg (g/dL) | -0.04 | 0.795 | 0.073 | 0.676 |
| ESR (mm/h) | 0.181 | 0.299 | 0.157 | 0.471 |
BMI: Body mass index; HbA1C: Haemoglubin A1C; FBS: Fasting blood sugar; LDL: Low density lipoprotein; HDL: High density lipoprotein; TG: Triglyceride; Chol: Cholesterol; Cr: Creatinine; Hg: Haemoglubin; ESR: Erythrocyte sedimentation rate. Only the variables that had a p<0.05 were considered in the final fitted model. Data were adjusted for age and BMI. r: Pearson’s correlation coefficient. (*significant correlation at the p<0.05 level)
A negative correlation was also observed between 25(OH)D and cholesterol in patients without DFU, where vitamin D decreased with increasing cholesterol levels (r= -0.401, p=0.017) [Table/Fig-3].
Discussion
In the present study, the levels of serum 25(OH)D and other biochemical parameters were evaluated in diabetic patients with or without foot ulcer and healthy individuals. Diabetic neuropathy and peripheral vascular disease are the main factors in DFU involvement. Over the recent years, many studies have been performed to examine the probable roles of 25(OH)D deficiency on pancreatic insulin release, immune system T-cells, inflammation and oxidative stress [8-10]. Some studies suggests that, diabetic neuropathy is one of the predisposing factors of foot ulcer and is probably related to vitamin D deficiency [11,12]. Our data showed that 25(OH)D deficiency may be related to DFU development. We further reported lower levels of 25(OH)D in patients with or without DFU as compared to controls. Zubair et al., also reported lower levels of 25(OH)D in patients with DFU compared to non-DFU patients [11]. Our findings were in accordance to Tiwari et al., study, which showed that, DFU patients had higher and more severe vitamin D deficiency as compared to non-DFU counterpart [12].
A double-blind, placebo-controlled randomised study, by Razzaghi et al., showed that vitamin D, compared with placebo, entailed a more significant improvement in wound parameters [4]. However, Afarideh et al., reported an unprecedented vitamin D increase in patients with chronic DFU involvement, probably owing to the selective alteration in the inflammatory status [5]. It has been shown that vitamin D stimulates phagocytosis and the killing of bacteria by macrophages [13]. Vitamin D also inhibits the proliferation of T cells and reduces production of cytokines by T helper type 1, while enhancing the production of T helper type 2 cytokines [14]. T- helper type 2 cells play a crucial role in responding to external pathogens (most bacteria and parasites) which may accelerate the wound healing [7,9]. Several recent studies have also underlined the role of hypovitaminose D in diabetes development [15–17]. The vitamin D receptor has been detected in pancreatic β-cells, in human and animal models. It has been shown that vitamin D impairs the synthesis and secretion of insulin, which suggests the role of vitamin D in type 2 diabetes mellitus development [16]. However, in our study, there was no significant difference in the level of vitamin D among patients without foot ulcer and healthy subjects. DFU development commonly occurs in diabetic patients with poor glycemic control or unidentified diabetic individuals over a long period of time [18]. And the results of our study showed significant differences in the measured biochemical parameters (such as HbA1C) among the groups. Tiwari et al., reported a higher HbA1C levels in patients with infectious DFU compared to non-infectious foot ulcer controls [12]. Additionally, higher incidence of DFU was observed in male patients as compared to females. In consistence with our data, Zhang et al. also reported a higher DFU prevalence in male type 2 diabetic patients as compared to females [19].
In the present research, DFU patients also showed the highest diabetes involvement period. Additionally, a positive correlation was observed between age and serum 25(OH)D level. In a study by Nasri et al., a positive correlation between age and vitamin D levels was demonstrated, indicating the importance of age consideration in vitamin D evaluation [20]. No consensus on the relationship between vitamin D and lipid profile was found. While some studies have indicated that vitamin D has an inverse relationship with triglyceride levels, other studies reported that the vitamin D deficient patients that higher TC/HDL ratio and 25(OH)D deficiency was prospectively associated with lower TC and HDL-C levels [21-22]. In our study, vitamin D levels in patients without DFU, showed a reverse relationship only with total cholesterol levels.
Limitation
The evaluation of different clinical and biochemical parameters in DFU and non-DFU diabetic patients and healthy controls were the strong points of the present study. However, larger study populations are required for more comprehensive analysis of various biochemical parameters.
Conclusion
The study indicates the possible correlation between low serum level of vitamin D and formation and progression of diabetic foot ulcers in type II diabetic patients. Thus highlighting the need for early estimation of serum 25(OH)D and vitamin D supplementation to improve glycemic index and prevent foot ulcers.
Data are presented as mean±SD or percent. ANOVA and post-hoc (Tukey) test were used for analysis. (ap<0.05 versus healthy individuals, bp<0.05 versus without foot ulcer group)
BMI: Body mass index; FBS: Fasting blood sugar; HbA1C: Haemoglubin A1C; LDL: Low density lipoprotein; HDL: High density lipoprotein; TG: Triglyceride; Chol: Cholesterol; Cr: Creatinine; Hg: Haemoglubin; ESR: Erythrocyte sedimentation rate; 25(OH)D: 25-hydroxy vitamin D
BMI: Body mass index; HbA1C: Haemoglubin A1C; FBS: Fasting blood sugar; LDL: Low density lipoprotein; HDL: High density lipoprotein; TG: Triglyceride; Chol: Cholesterol; Cr: Creatinine; Hg: Haemoglubin; ESR: Erythrocyte sedimentation rate. Only the variables that had a p<0.05 were considered in the final fitted model. Data were adjusted for age and BMI. r: Pearson’s correlation coefficient. (*significant correlation at the p<0.05 level)
[1]. American Diabetes AssociationDiagnosis and classification of diabetes mellitusDiabetes Care 2010 33(Suppl 1):S62-9.doi: 10.2337/dc10-S06210.2337/dc10-S06220042775 [Google Scholar] [CrossRef] [PubMed]
[2]. Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040Diabetes Res Clin Pract 2017 128:40-50.doi: 10.1016/j.diabres.2017.03.02410.1016/j.diabres.2017.03.02428437734 [Google Scholar] [CrossRef] [PubMed]
[3]. Esteghamati A, Larijani B, Aghajani MH, Ghaemi F, Kermanchi J, Shahrami A, Diabetes in Iran: Prospective Analysis from First Nationwide Diabetes Report of National Program for Prevention and Control of Diabetes (NPPCD-2016)Sci Rep 2017 7(1):13461doi: 10.1038/s41598-017-13379-z10.1038/s41598-017-13379-z29044139 [Google Scholar] [CrossRef] [PubMed]
[4]. Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trialJ Diabetes Complications 2017 31(4):766-72.doi: 10.1016/j.jdiacomp.2016.06.01710.1016/j.jdiacomp.2016.06.01727363929 [Google Scholar] [CrossRef] [PubMed]
[5]. Afarideh M, Ghanbari P, Noshad S, Ghajar A, Nakhjavani M, Esteghamati A, Raised serum 25-hydroxyvitamin D levels in patients with active diabetic foot ulcersBr J Nutr 2016 115(11):1938-46.doi: 10.1017/S000711451600109410.1017/S000711451600109427153203 [Google Scholar] [CrossRef] [PubMed]
[6]. de Haan K, Groeneveld ABJ, de Geus HRH, Egal M, Struijs A, Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysisCrit Care 2014 18(6):660doi: 10.1186/s13054-014-0660-410.1186/s13054-014-0660-425475621 [Google Scholar] [CrossRef] [PubMed]
[7]. Upala S, Sanguankeo A, Permpalung N, Significant association between vitamin D deficiency and sepsis: a systematic review and meta-analysisBMC Anesthesiol 2015 15:84doi: 10.1186/s12871-015-0063-310.1186/s12871-015-0063-326041306 [Google Scholar] [CrossRef] [PubMed]
[8]. Karadurmus N, Sahin M, Tasci C, Naharci I, Ozturk C, Ilbasmis S, Potential benefits of hyperbaric oxygen therapy on atherosclerosis and glycaemic control in patients with diabetic footEndokrynol Pol n.d. 61(3):275-9. [Google Scholar]
[9]. Sytze Van Dam P, Cotter MA, Bravenboer B, Cameron NE, Pathogenesis of diabetic neuropathy: focus on neurovascular mechanismsEur J Pharmacol 2013 719(1-3):180-6.doi: 10.1016/j.ejphar.2013.07.01710.1016/j.ejphar.2013.07.01723872412 [Google Scholar] [CrossRef] [PubMed]
[10]. Lv WS, Zhao WJ, Gong SL, Fang DD, Wang B, Fu ZJ, Serum 25-hydroxyvitamin D levels and peripheral neuropathy in patients with type 2 diabetes: a systematic review and meta-analysisJ Endocrinol Invest 2015 38(5):513-8.doi: 10.1007/s40618-014-0210-610.1007/s40618-014-0210-625527161 [Google Scholar] [CrossRef] [PubMed]
[11]. Zubair M, Malik A, Meerza D, Ahmad J, 25-Hydroxyvitamin D [25(OH)D] levels and diabetic foot ulcer: is there any relationship?Diabetes Metab Syndr n.d. 7(3):148-53.doi: 10.1016/j.dsx.2013.06.00810.1016/j.dsx.2013.06.00823953180 [Google Scholar] [CrossRef] [PubMed]
[12]. Tiwari S, Pratyush DD, Gupta B, Dwivedi A, Chaudhary S, Rayicherla RK, Prevalence and severity of vitamin D deficiency in patients with diabetic foot infectionBr J Nutr 2013 109(1):99-102.doi: 10.1017/S000711451200057810.1017/S000711451200057822715859 [Google Scholar] [CrossRef] [PubMed]
[13]. van Etten E, Decallonne B, Bouillon R, Mathieu C, NOD bone marrow-derived dendritic cells are modulated by analogs of 1,25-dihydroxyvitamin D3J Steroid Biochem Mol Biol 2004 89-90(1-5):457-9.doi: 10.1016/j.jsbmb.2004.03.01710.1016/j.jsbmb.2004.03.01715225820 [Google Scholar] [CrossRef] [PubMed]
[14]. van Etten E, Mathieu C, Immunoregulation by 1,25-dihydroxyvitamin D3: basic conceptsJ Steroid Biochem Mol Biol 2005 97(1-2):93-101.doi: 10.1016/j.jsbmb.2005.06.00210.1016/j.jsbmb.2005.06.00216046118 [Google Scholar] [CrossRef] [PubMed]
[15]. Wu-Wong JR, Potential for vitamin D receptor agonists in the treatment of cardiovascular diseaseBr J Pharmacol 2009 158(2):395-412.doi: 10.1111/j.1476-5381.2009.00171.x10.1111/j.1476-5381.2009.00171.x19371337 [Google Scholar] [CrossRef] [PubMed]
[16]. Mathieu C, Gysemans C, Giulietti A, Bouillon R, Vitamin D and diabetesDiabetologia 2005 48(7):1247-57.doi: 10.1007/s00125-005-1802-710.1007/s00125-005-1802-715971062 [Google Scholar] [CrossRef] [PubMed]
[17]. Hutchinson MS, Grimnes G, Joakimsen RM, Figenschau Y, Jorde R, Low serum 25-hydroxyvitamin D levels are associated with increased all-cause mortality risk in a general population: the Tromsø studyEur J Endocrinol 2010 162(5):935-42.doi: 10.1530/EJE-09-104110.1530/EJE-09-104120185562 [Google Scholar] [CrossRef] [PubMed]
[18]. Zemel MB, Nutritional and endocrine modulation of intracellular calcium: implications in obesity, insulin resistance and hypertensionMol Cell Biochem 1998 188(1-2):129-36.10.1007/978-1-4615-5763-0_149823018 [Google Scholar] [CrossRef] [PubMed]
[19]. Zhang P, Lu J, Jing Y, Tang S, Zhu D, Bi Y, Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis †Ann Med 2017 49(2):106-16.doi: 10.1080/07853890.2016.123193210.1080/07853890.2016.123193227585063 [Google Scholar] [CrossRef] [PubMed]
[20]. Nasri H, Ardalan MR, Association of serum vitamin D level with age in individuals with normal renal functionJ Nephropharmacology 2012 1(1):7-9. [Google Scholar]
[21]. Sung-Kyu Choi, Jin-A Park, Sang-Keun Ham, Ji-Young Yoon, Sang-Min Kim, Jin-Young Kim U-TK, Association between Vitamin D and Serum CholesterolKorean J Fam Pr 2015 5(4):441-6. [Google Scholar]
[22]. Faridi KF, Zhao D, Martin SS, Lupton JR, Jones SR, Guallar E, Serum vitamin D and change in lipid levels over 5 y: The Atherosclerosis Risk in Communities studyNutrition 2017 38:85-93.doi: 10.1016/j.nut.2017.01.00810.1016/j.nut.2017.01.00828526388 [Google Scholar] [CrossRef] [PubMed]