Cancer is one of the most common causes of morbidity and mortality today, with more than 10 million new cases and more than six million deaths each year worldwide. More than 20 million persons around the world live with a diagnosis of cancer and more than half of all cancer cases occur in the developing countries [1]. Based on cancer statistics, oral cancer is a major problem in India and accounts for 50-70% of all cancers diagnosed every year [2]. Sociodemographic, socioeconomic and lifestyle factors are influencing the pathogenesis of oral cancer [3]. In developing countries, a high proportion of oral cancer subjects were from lower socioeconomic classes and advanced clinical TNM Stages III and IV [4]. Oral squamous cell carcinoma is aggressive in nature. Although advanced techniques are available, the five years survival rate is approximately 50% [5]. ECOG-PS scale was used as a global assessment tool for the cancer subject’s physical functioning and the ability of the self-care test [6]. As Kelly CM and Shahrokni A, reported that accurate measurement of subjects physical fitness for treatment would help oncologists to select the most appropriate treatment options which reduce toxicity and improves survival outcome [7]. Though, ECOG-PS was the quite an old prognostic tool for cancer. The recent prospective study from the regional cancer centre, Karnataka evaluated the efficacy and toxicity of 5-fluorouracil and cisplatin versus taxane and cisplatin as induction chemotherapy in advanced head and neck squamous cell carcinoma based on ECOG-PS [8]. Similarly, another study from Tata memorial cancer centre, Maharashtra, India has also conducted weekly induction of chemotherapy in head and neck squamous cell carcinoma subjects based on ECOG-PS [9]. Thus, the recent research is focusing the ECOG-PS because physical fitness is an important tool for the prediction of survival. Therefore, the present study was intended to evaluate the association of ECOG performance score with sociodemographic, socioeconomic, clinicopathological characteristics and survival outcome of buccal mucosa squamous cell carcinoma.
Materials and Methods
This prospective study was conducted in the regional cancer centre, Arignar anna memorial cancer hospital and research centre, Kanchipuram, Tamil Nadu, India. The Institutional Ethical Committee permission was obtained from the Directorate of Medical Education (DME), Tamil Nadu to conduct the study (No.24984/2013). A total of 236 subjects were estimated using z=1.95 at 5% level of significance, p=57.3% survival rate of oral squamous cell carcinoma, d=6% margin of error based on previous publication [10]. The present study assessed a total of 198 buccal squamous cell carcinoma subjects between March 2013-January 2015 due to study limitation and those were followed-up until January 2016.
Data collection methods: A total of 198 clinicopathologically confirmed buccal mucosa squamous cell carcinoma subjects were included in the study whereas precancerous conditions and other oral subsites were excluded from the study. Sociodemographic factors including gender, age, Body Mass Index (BMI), risk habits and socioeconomic status which include details of education, occupation and family income according to kuppuswamy’s modified scale were collected; clinical features like tumour cell differentiation, TNM stage, nodal and metastasis status according to Union for International Cancer Control (UICC) and their follow-up details were retrieved from hospital registries [11,12]. ECOG-PS was collected based on the physical performance of subjects at the time of diagnosis, which is composed of five categories [7]. In the present study, authors divided buccal mucosa squamous cell carcinoma subject’s into two groups according to the ECOG-PS score (ECOG 0-1, good performance score and ECOG 2-4, poor performance score) as previously reported by Batista Correa GF et al., [13]. The entire subjects were regularly followed at three months interval. For the study, between March 2013 and January 2016 period, follow-up details were collected from medical registries, whoever missed follow-up at last visit, details were collected from the phone. Survival Outcome: Overall survival was defined as the time from the first day of treatment to date of death, censored at the date last known alive [14].
Statistical Analysis
The statistical analyses were assessed using the SPSS 16.0 package (SPSS Inc., Chicago, USA). Pearson’s chi-square (χ2) test and binary logistic regression analysis were performed to evaluate the association between the covariates and the ECOG-PS score. For survival analysis, Kaplan-Meier and the log-rank test were used to analyse differences between survival probabilities. All statistical significance was set at p<0.05.
Results
Subject’s characteristics: The present study depicted ECOG-PS based on the physical performance of buccal mucosa squamous cell carcinoma subjects. [Table/Fig-1] shows the ECOG-PS scoring scale procedure which consisted of five categories. Of 198 subjects, 167 (84.4%) subjects were recorded with poor performance score (i.e., ECOG 2-4) whereas rest of 31 (15.6%) subjects were recorded with good performance score (i.e., ECOG 0-1). [Table/Fig-2] illustrates the association between sociodemographic, socioeconomic and clinicopathological features with ECOG performance score of subjects.
ECOG Performance Status scoring scale.
| Grade | ECOG |
|---|
| 0 | Fully active, able to carry on all pre-disease performance without restriction. |
| 1 | Restricted in physically strenuous activity but ambulatory and able to carry out work of light or sedentary nature. |
| 2 | Ambulatory and capable of all selfcare but unable to carry out any work activities. Up and about more than 50% of walking hours. |
| 3 | Capable of only limited selfcare, confined to bed or chair more than 50% of walking hours. |
| 4 | Completely disabled cannot carry on any selfcare. Totally, confined to bed or chair. |
| 5 | Dead. |
Analysis of sociodemographic, socioeconomic and clinicopathologic features according to ECOG-PS in the study population.
| Subjects Characteristics | n=198 | ECOG-PS | p-value |
|---|
| Good (0-1) n=31 | Poor (2-4) n=167 |
|---|
| Sociodemographic |
| Gender | Male | 125 | 21 (67.7) | 104 (62.3) | 0.562 |
| Female | 73 | 10 (32.3) | 63 (37.7) |
| Age | <40 | 71 | 15 (48.4) | 56 (33.5) | 0.113 |
| ≥40 | 127 | 16 (51.6) | 111 (66.5) |
| Body mass Index (BMI) | Underweight | 89 | 13 (41.9) | 76 (45.5) | 0.971 |
| Healthy weight | 50 | 8 (25.8) | 42 (25.1) |
| Over weight | 39 | 7 (22.6) | 32 (19.2) |
| Obese | 20 | 3 (9.7) | 17 (10.2) |
| Risk habits | Tobacco | 165 | 26 (83.9) | 139 (83.2) | 0.93 |
| Non-tobacco | 33 | 5 (16.1) | 28 (16.8) |
| Socioeconomic status | Upper | 5 | 4 (12.9) | 1 (0.6) | ≤0.001* |
| Upper middle | 16 | 6 (19.4) | 10 (6) |
| Lower middle | 21 | 4 (12.9) | 17 (10.2) |
| Lower upper | 32 | 4 (12.9) | 28 (16.8) |
| Lower | 124 | 13 (41.9) | 111 (66.5) |
| Clinico-pathologic features |
| Cell differentiation | Well | 104 | 13 (41.9) | 91 (54.5) | 0.034* |
| Moderate | 66 | 9 (29) | 57 (34.1) |
| Poor | 28 | 9 (29) | 19 (11.4) |
| TNM stage | Stage I | 13 | 13 (41.9) | 0 | ≤0.001* |
| Stage II | 17 | 17 (54.8) | 0 |
| Stage III | 13 | 1 (3.2) | 12 (7.2) |
| Stage IV | 155 | 0 | 155 (92.8) |
| Lymphnode status | Negative | 30 | 30 (96.8) | 0 | ≤0.001* |
| Positive | 168 | 1 (3.2) | 167 (100) |
| Metastasis | Negative | 141 | 31 (100) | 110 (65.9) | ≤0.001* |
| Positive | 57 | 0 | 57 (34.1) |
*The statistical significance at p<0.05 by chi-square method
Association of demographic characteristics: The present study includes 198 buccal mucosa squamous cell carcinoma subjects which comprised 125 (63.1%) male and 73 (36.9%) female in 1.7:1, respectively. Of 198 subjects, the most frequent of subject’s 127 (51.6%) were enrolled from elders (≥40 years). The mean age of subjects was 54.16±17.25 (mean±SD) years, range from 21 to 88 years. In the present study, all the subjects were reported with either tobacco (smoking and smokeless form) or non-tobacco (betal nut/pan masala) chewing habits. However, most of the subjects 165 (83.3%) were identified as tobacco habitual. The median BMI was 20.4 Kg/m2. The most of subject’s i.e., 89 was diagnosed with underweight i.e., below 18 Kg/m2, those might be severely affected by malnutrition. However, the present study revealed a non-significant relation between demographic characteristics and ECOG-PS by chi-square analysis at p<0.05 [Table/Fig-2].
Association of socioeconomic status: The present study illustrated that 124 (62.6%) subjects were from lower Socioeconomic Status (SES) and followed by other groups. In lower SES status, 111 (66.5%) had reported with lower physical function of subjects (poor ECOG performance score). However, the socioeconomic status showed a highly significant association at p<0.05 [Table/Fig-2].
Association of clinico-pathological characteristics: The histopathology features are an important prognostic factor for buccal mucosa squamous cell carcinoma. In the study, 168 (84.8%) had presented at the advanced stage of tumour, whoever could not take self-care at the time of diagnosis. The cell differentiation (p=0.034), TNM stage, nodal and metastasis status had a highly significant difference in ECOG-PS at p<0.05 [Table/Fig-2]. Further, binary logistic regression also revealed significant nodal (p=0.032) and metastasis status (p=0.049) of subjects at 95 CI, p<0.05. The present study reports that using status of ECOG-PS, the presence of lymph node and metastasis had 2.27 fold (95CI, 1.551-4.540) and 1.053 (95CI, 0.534-2.242) risk of disease recurrence/death of subjects than negative lymph node and metastasis in subjects, respectively [Table/Fig-3].
Binary logistic regression analysis of the covariates investigated according to the ECOG-PS of subjects.
| Subjects Characteristics | ECOG-PS | p-value |
|---|
| OR | 95 (CI) |
|---|
| Lymphnode status | Negative | 1 | | |
| Positive | 2.27 | 1.551-4.540 | 0.032* |
| Metastasis | Negative | 1 | | |
| Positive | 1.053 | 0.534-2.242 | 0.049* |
*Statistical significant considered at p<0.05 level
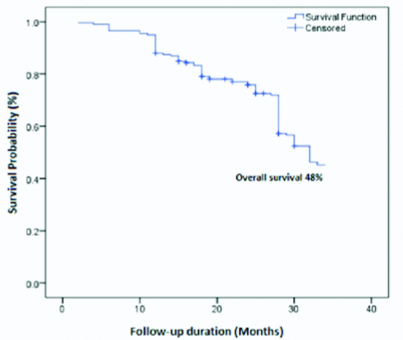
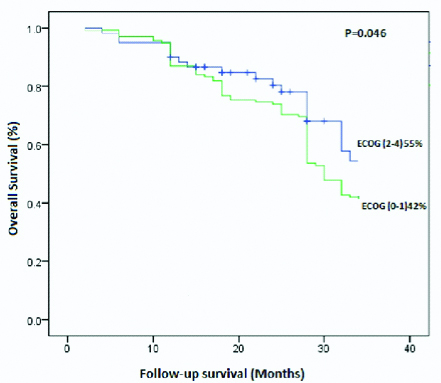
Association of survival outcome: A total of 198 subjects were followed-up for an average of 18 months. Of 198 subjects, 7 (3.5%) found with disease-specific death, 24 (12.1%) had reported with recurrence and rest of subjects 167 (84.4%) were alive without any disease symptoms at last follow-up. The [Table/Fig-4] shows the estimated approximately three years overall survival (48%) of buccal mucosa squamous cell carcinoma subjects by Kaplan-Meier analysis. The [Table/Fig-5] showed an overall survival difference among the ECOG-PS by Kaplan-Meier analysis using the log-rank test (p=0.046, p<0.05). The good physical ECOG performance status had a better prognosis (55%) than the poor performance score of ECOG (42%). Thus, a good physical performance score (1-2) improves a better survival outcome.
Kaplan-Meier overall survival curves of buccal mucosa squamous cell carcinoma subjects.
Kaplan-Meier overall survival curves of buccal mucosa squamous cell carcinoma subjects based on ECOG performance score.
Discussion
Despite continued advancement in cancer treatment, many cases of cancer remain incurable. Accurate prognostic information could help the physicians to decide anticancer therapy and enable appropriate advance care planning and end of life decision making [6].
A recent study was conducted to analyse the prognosis of ECOG-PS in pancreatic cancer. They selected poor ECOG-PS (2-4) pancreatic cancer subjects for palliative care and good ECOG-PS score (0-1) for curative intent (0-1) and proved ECOG-PS could be a best prognostic tool for pancreatic cancer subjects [15]. Another chemotherapy study has also followed a dose of drug level based on ECOG-PS and proved it could be a prognostic factor for gastric cancer subjects [16]. In contrary, a retrospective study reported that ECOG-PS does not affect the survival outcome of non-Hodgkin’s lymphoma subjects [17]. However, the present study showed the survival difference based on the physical fitness of subjects using ECOG score system.
ECOG reported that socioeconomic inequalities were a risk factor for total mortality and for many causes of death [18]. Batista Correa GF et al., conducted a study of the association of demographic, socioeconomic and clinical factors and ECOG-PS of head and neck squamous cell carcinoma. There study identified that ECOG-PS was a simple prognostic tool in head and neck squamous cell carcinoma and showed a significant difference of sociodemographic, socioeconomic factors and clinical features [13]. Another recent study also supported that the similar survival difference of sociodemographic factors such as age and gender reported in advanced stage non-small cell lung squamous cell carcinoma based on ECOG-PS with bevacizumab chemotherapy clinical trial [19]. In accordance with previous reports, the present study also supported that ECOG-PS showed a highly significant association with the socioeconomic status of subjects. However, the demographic characteristics of age, gender and BMI failed to show significant relevance with ECOG-PS. Thus, the demographic character does not account for ECOG-PS of subjects.
The recent cancer research is focusing based on the physical performance and clinicopathological features of subjects. Traditionally, a clinicopathologic feature like tumour size, depth, nodal involvement, metastasis and invasion were used as the prognostic factor for oral cancer [20]. The clinicopathological features of tumour size, the presence of lymph node metastasis were an independent association with ECOG performance score in head and neck squamous cell carcinoma [13]. The present results also support with previous results, the clinicopathological features nodal and metastasis status had a high significant difference based on ECOG-PS. Moreover, the present study revealed that low survival of poor ECOG-PS than good ECOG-PS of buccal mucosa squamous cell carcinoma subjects.
Limitation
Actually, this is a very small study, that too single institute regional cancer centre which is located in rural area of India.
Conclusion
The present study concludes that the subject’s physical performance score using ECOG-PS scale at diagnosis may help to identify the severity of disease and may help medical care requirements, specific therapeutic and rehabilitative interventions.
*The statistical significance at p<0.05 by chi-square method
*Statistical significant considered at p<0.05 level