Approximately 2-3% of all live births are affected by congenital malformations. CNS anomalies, whether they present as isolated or part of syndromes, are the second most frequent serious congenital anomaly, after congenital heart diseases [1]. Hence, various imaging techniques are needed for proper diagnosis.
Cranial ultrasonography is the most common technique used for routine evaluation of cerebral morphology and lesions in neonates. It has also been widely used in infants, particularly in those who are at risk for brain lesions, such as those born prematurely, those with abnormal neurological signs or with history of birth asphyxia [2]. Brain abnormalities have also been reported in apparently healthy neonates, with only mild to moderate impairment of neurological development [3]. Therefore, it is being used as a screening test in some centers [4].
As the procedure is non-invasive as well as safe and cost-effective, serial scans can be performed for dynamic evaluation of the lesions. Likewise, cranial ultrasound has significantly improved the diagnosis of brain lesions and also the outcome of the patients. However, even though ultrasound can accurately diagnose major brain anomalies, subsequent MRI and CT may be needed for more detailed imaging [5].
Through this study we aimed to evaluate the frequency and type of brain abnormalities detected by cranial ultrasound in neonates and infants in our center. Moreover, we aimed to evaluate the impact of prematurity and postnatal events in the development of CNS abnormalities, as well as the rate of prenatal diagnosis.
Materials and Methods
A retrospective review of the data of infants, who underwent cranial ultrasound between January 2010 and December 2017, in the Department of Paediatric Neurology in the Paediatric Clinic and also in the Neonatology Clinic, University Clinical Centre of Kosovo, was conducted. The patient population included preterm newborns, as well as full-term newborns with perinatal asphyxia, respiratory distress, neurological deficiency, suspected syndromes and other congenital anomalies. All infants were examined in supine position and standard coronal and sagittal images of the brain through the anterior fontanelle were obtained using 5 to 8 MHz transducers (Acuson x300 ultrasound system, Siemens, Germany). All scans were performed or confirmed by a single experienced Pediatric Neurologist. Study was approved by local Ethics committee and informed consents were obtained (reference number 88/17). The following data were collected: date of birth, gestational age, delivery mode, Apgar score on 1st and 5th minute, birth weight, presence of jaundice, neurological symptoms, head circumference, multiple pregnancy, maternal history (maternal drug use, congenital infections or gestational diabetes mellitus) and family history.
More significant abnormalities such as agenesis of the corpus callosum, haemorrhages, ventriculomegaly or hydrocephalus, hypoxic-ischemic injury and abnormal parenchymal echogenicity were considered as major. All infants with major brain lesions underwent further clinical and imaging evaluation through Magnetic Resonance Imaging (MRI) and/or serial sonographic scans.
Results
During the study period 73259 neonates were born in the University Clinical center of Kosovo. Amongst them, selective cranial ultrasound was performed in 4256 (5.8%) infants. Sonographic abnormalities were detected in 371 (8.7%) of them (52% males and 48% females). Findings are summarized in [Table/Fig-1]. Major abnormalities were found in 245 (of 371, i.e., 66%) patients.
The frequency of different brain anomalies through years.
| Diagnosis | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Total | Incidence |
|---|
| Brain tumor | | 1 | | | | | 1 | | 2 | 0.027:1000 |
| Agenesis of cavum septum pellucidi | | | | 1 | | | | | 1 | 0.013:1000 |
| Lisencephaly | | | | | | | 1 | | 1 | 0.013:1000 |
| Intracranial calcifications | | | | | 2 | | 1 | | 3 | 0.04:1000 |
| Cyst of the pellucid cavum | | | | 2 | | | 2 | | 4 | 0.054:1000 |
| Vein of Galen aneurysm | | | | | 3 | | 1 | 1 | 5 | 0.068:1000 |
| Agenesis of the corpus callosum | 1 | | | 2 | 2 | 1 | 2 | 2 | 10 | 0.13:1000 |
| Partial agenesis of corpus callosum | 3 | | 1 | 2 | 2 | 4 | | 2 | 14 | 0.19:1000 |
| Cyst of choroidal plexus | 2 | | 2 | 1 | 1 | 2 | 1 | 11 | 20 | 0.27:1000 |
| Cyst of the germinal matrix | 6 | 2 | 3 | 4 | 2 | 4 | 1 | 3 | 25 | 0.34:1000 |
| Ventriculomegaly | 9 | 5 | 7 | 6 | 9 | 4 | 11 | 8 | 59 | 0.8:1000 |
| Internal hydrocephalus | 7 | 12 | 6 | 18 | 10 | 12 | 7 | 5 | 77 | 1.05:1000 |
| External hydrocephalus | | 1 | 2 | | 1 | | | 1 | 5 | 0.068:1000 |
| Porencephaly | 4 | 1 | 2 | 3 | 4 | 2 | 1 | 3 | 20 | 0.27:1000 |
| Periventricular leukomalacia | 9 | 8 | 11 | 11 | 6 | 6 | 5 | 4 | 60 | 0.82:1000 |
| Intracranial haemorrhage | 5 | 9 | 3 | 7 | 6 | 8 | 4 | 8 | 50 | 0.68:1000 |
| Dandy Walker | 1 | | | 1 | | | | 1 | 3 | 0.04:1000 |
| Arnold Chiari malformation | 1 | | 2 | 2 | | 3 | 4 | | 12 | 0.16:1000 |
| Births | 10606 | 10567 | 10716 | 10426 | 10422 | 10414 | 10202 | 10328 | 73259 | 5.06:1000 |
Intracranial Haemorrhages (ICH) were found in 50 neonates. Amongst them 36 were prematures {median gestational weeks 32 (26-36)}. Two full-term newborns presented large for gestational age and needed forceps assistance at delivery. The other 12 had low Apgar score [Table/Fig-1]. Amongst all patients with ICH, seven of them had low thrombocyte count, less than 100×10-9/L. Brain tumour was found in two infants, age 8 and 12 months, that presented with increased head circumference and abnormal neurological development.
Lisencephaly was found in a two-week-old baby girl that manifested with generalised hypotonia and seizures.
Intracranial calcifications were found in three newborns, two of them presented with multiple anomalies, and the third one was a premature infant who presented with hypotonia and seizures. TORCH test resulted positive for Cytomegalovirus in all three cases.
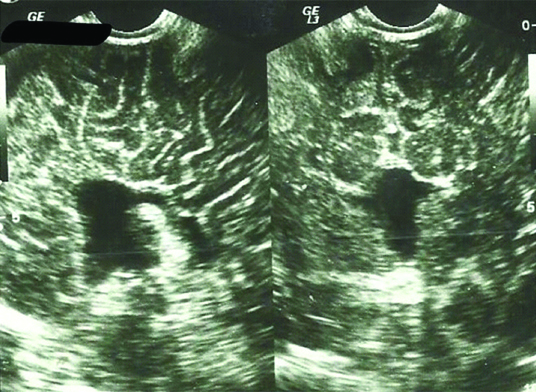
Agenesis of the corpus callosum was found in ten patients. Seven patients had associated brain anomalies (two ventriculomegalies, one Dandy-Walker malformation, one Arnold-Chiari malformation and three porencephaly) and presented in first month of life with generalized hypotonia, irritability, increased head circumference or seizures. The other three presented after six months of life with neurological development delays as well as seizures in one of them [Table/Fig-2].
Agenesis of corpus callosum in sagittal and coronal planes.
Isolated ventriculomegaly was found in 59 cases. All patients had a history of intracranial haemorrhage during perinatal period or meningitis.
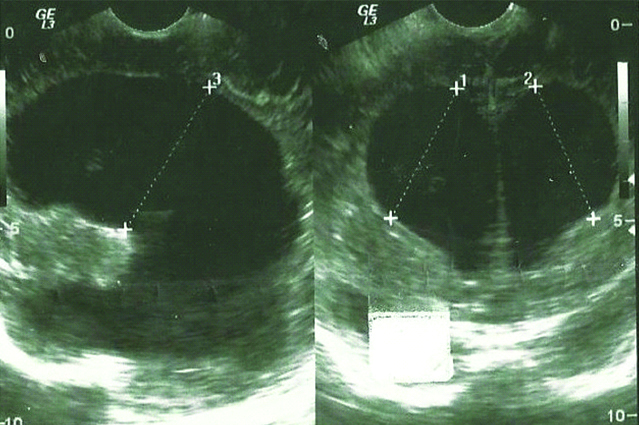
Hydrocephalus was found in 82 patients, median age 2 (1-9) months. Amongst them, five were found to have external hydrocephalus, median age 2 (1-4) months. A 53% of cases had associated CNS anomalies [Table/Fig-3].
Severe form of Hydrocephalus.
Porencephaly was noticed in 20 patients. Thirteen of them were considered to have unilateral encephaloclastic porencephaly which is usually caused by intracerebral haemorrhage or infection in the third trimester of pregnancy. Only three of them had prenatal diagnosis.
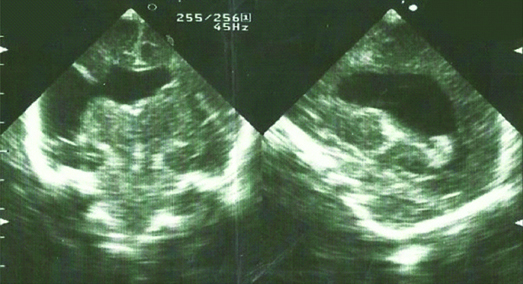
Schizencephalic porencephaly was considered to be the diagnosis of the other seven patients manifesting as a grey matter lined cleft extending from the ependyma to the pia mater [Table/Fig-4]. Three of them had bilateral changes and other associated congenital anomalies (two hydrocephalus, one orofaciodigital syndrome type I). All patients had clinical changes such as hypotonia, irritability, poor feeding and seizures.
Unilateral schizencephaly.
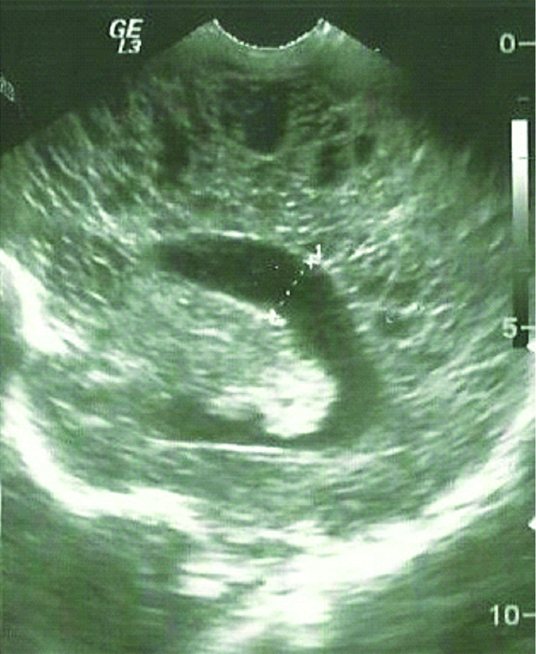
Periventricular leukomalacia was considered as a diagnosis in 60 patients. All of them had a history of prematurity with perinatal asphyxia and low Apgar score [Table/Fig-5,6].
General characteristics of the patients with major brain anomalies.
| Diagnosis | No | Age | Gender F (%) | SGA | LGA | Head circumference z-score | Mean Apgar score 1/5 min | Prenatal diagnosis | Gestational weeks |
|---|
| Brain tumor | 2 | 8, 12 months | 0 | 0 | 0 | 2.3 and 2.5 | 8/9 | 0 | 39, 39 |
| Intracranial haemorrhage | 50 | 3 (1-21) days | 46% | 0 | 2 | 0.34 (-0.8 to 1.2) | 6.1/ 7.8 | 0 | 32 (26-38) |
| Agenesis of corpus callosum | 10 | 2 (1-9) months | 60% | 1 | 0 | 0.45 (-0.3 to 1.7) | 8.2/9 | 0 | 38 (37-40) |
| Hydrocephalus | 82 | 2 (1-9) months | 52% | 2 | 4 | 2.3 (1.7 to 4.1) | 6.8/7.8 | 0 | 34 (30-41) |
| Periventricular leukomalacia | 60 | 3 (1-6) months | 40% | 1 | 2 | 0.3 (-0.1 to 0.6) | 5.8/7.4 | 0 | 31 (25-40) |
| Porencephaly | 20 | 19 (1-30) days | 54% | 1 | 0 | -0.2 (-0.7 to 1.2) | 7.2/8.3 | 3 | 34 (32-40) |
| Vein of Galen aneurysm | 5 | 2 (1-4) weeks | 60% | 0 | 0 | 1.2 (0.5-2.5) | 7.9/8.8 | 0 | 38 (37-40) |
| Lisencephaly | 1 | 2 weeks | 100% | 0 | 0 | 0.4 | 6/7 | 0 | 39 |
| Dandy Walker | 3 | 2 (1-4) months | 33% | 0 | 0 | 2.3 (2.1-2.7) | 6.3/8 | 0 | 38 (36-40) |
| Arnold Chiari malformation | 12 | 1 (1-3) days | 43% | 1 | 1 | 1.1 (0.7-2.3) | 6.4/8.5 | 0 | 38 (36-40) |
Sagittal scan in a three-week-old premature neonate showing multiple periventricular cysts typical of this stage of periventricular leukomalacia.
Dandy-Walker malformation was found in three patients. Age at presentation was one, two and four months respectively. All patients presented with increased head circumference due to hydrocephalus.
Arnold-Chiari malformation type II was found in twelve neonates. All patients had associated myelomeningocele. Poor feeding and dyspnea were the main clinical features. All patients went magnetic resonance imaging for final diagnosis.
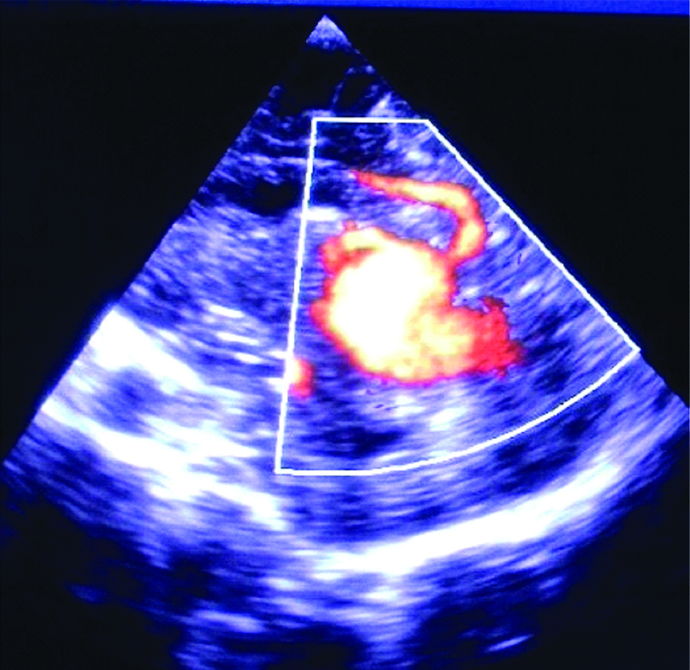
Vein of Galen malformation was found in five patients in the first month of life. Three of them manifested with congestive heart failure, and the other two had increased cranial circumference and prominent scalp veins due to hydrocephalus [Table/Fig-7].
Sagittal scan showing vein of Galen malformation.
Discussion
Using the standard views through anterior fontanel, incidence was quite variable among different studies. Wang LW et al., reported a very low incidence (0.25%) of abnormal head ultrasound findings on 2309 patients [6], while Heibel M et al., reported a 9% abnormal findings in 1000 asymptomatic neonates [7]. As expected, usage of the MRI increases the number of patients with abnormal brain findings [8]. Furthermore, anomalies of the brain encompass different aetiologic factors such as genetic, infective, teratogenic as well as events during pre, peri and post-natal period. Hence, it is logical that the incidence of brain anomalies varies among countries [9]. In the present series, intracranial haemorrhages, hydrocephalus, Periventricular leukomalacia and porencephaly predominated amongst major anomalies.
ICH is one the most common cause of mortality and neurologic sequels in the newborns. Similar to other studies the rate of ICH was significantly higher in preterm infants compared to the ones on term. In newborns with birth weight less than 1500 g the incidence is around 25-40% [10]. In term infants, the reported incidence of intracranial haemorrhages ranges from 10% in symptomatic infants with neurological signs or pulmonary diseases [11] to 0.09% in apparently normal infants [6].
The incidence of intracranial haemorrhage in this series was 0.68:1000 and similar to other studies it was associated with birth trauma, perinatal asphyxia, pulmonary diseases, systemic or pulmonary hypertension, haemorrhagic diatheses [12-14].
Corpus callosum agenesis is found in 2-3% of children with abnormal neurologic developmental [15]. In this study the incidence of complete agenesis was 0.13:1000, while partial agenesis incidence was 0.19:1000. The most common neurological abnormalities in patients with corpus callosum agenesis are mental retardation or cerebral palsy in 82% and 31% of cases, respectively [16]. In the present study three children with isolated corpus callosum agenesis presented after six months of life with motor impairment, and seizures in one of them, despite asymptomatic neonatal periods. Similar to our study, the agenesis of corpus callosum is associated with other brain anomalies in 80% of cases [17].
Usual exogenous causes are congenital rubella, alcoholic fetal syndrome and insulin-dependent diabetes of the mother. However, since this is a retrospective study, we had limited data about maternal history of our infants. Diagnosis is established through ultrasound, where we can notice divergent frontal horns similar to bat’s wings as well as widening of the occipital wings of the lateral ventricle named colpocephalia. Third ventricle is often dilated. Also, there is radial alignment of the brain gyri [Table/Fig-2].
On the other hand, partial agenesis of the corpus callosum presents at later stages of embryonal development. It can affect anterior or posterior part of the corpus callosum.
Cytomegalovirus is the most common organism causing congenital central nervous system infection. Earlier infection might affect organogenesis resulting in multiple anomalies, while later infections usually cause destructive changes with calcifications [2].
Ventriculomegaly is the most common congenital CNS anomaly (0.03-0.15%) [18]. Association with other congenital or acquired CNS anomalies is common. In the present series, 30% of congenital CNS anomalies (Dandy Walker malformation, corpus callosum agenesis, arachnoid cysts) had associated ventriculomegaly. On the other hand, haemorrhages or infections were speculated as a cause in the rest of patients with isolated ventriculomegaly [19,20].
Hydrocephalus is defined as severe ventriculomegaly with atrial width of more than 20 mm [21]. In this series, 77 (1.05:1000) patients were found to have hydrocephalus.
Porencephaly prevalence is about 0.1-0.2 in 1000 newborns [22], similar to present series (0.17:1000). Based on aetiology porencephaly might be divided in two types. Encephaloclastic porencephaly (type I) occurs as a consequence of brain damage followed by liquefaction of the brain tissue, while schizencephalic porencephaly (type II) is caused by abnormal neural migration and cortical organization. Type I is usually unilateral and more frequent, similar to our series of patients (10 out of 13). Type II is less frequent and usually associated with other anomalies and has poorer prognosis [22].
Periventricular Leukomalacia (PVL) is the most common ischemic brain injury in premature infants. Initial ultrasonography shows abnormal increased echogenicity of the periventricular white matter, while periventricular cysts might develop in approximately 15% of infants, 2-3 weeks after [Table/Fig-5].
Dandy-Walker malformation was found in three patients. Similar to our study population, its incidence is reported to be about 1 in 25,000-35,000 births [23]. Defective structures originating from rhomboencephalic roof result in development of Dandy-Walker malformation [23,24]. This leads to cystic dilatation of the fourth ventricle compressing the cisterna magna, hence hydrocephalus development. Hydrocephalus was found in all three patients.
Arnold-Chiari malformation type II was found in twelve neonates, all patients had associated myelomeningocele. Hence, cranial ultrasound should always be performed in neonates with spinal dysraphisms. According to D’Addario V et al., some degree of Arnold-Chiari type II malformation in presented in all infants with spina bifida [25].
Vein of Galen malformation is a form of embryonic arteriovenous shunt. Hence, increased cardiac preload due to the ’steal phenomenon’ might cause heart failure, as manifested in three (60%) of our patients. Sometimes it might compress the aqueduct, hence secondary hydrocephalus development [26].
Limitation
This study had certain limitations. First, this was a retrospective study. Second, ultrasound was performed only in symptomatic patients, while it was known that there are abnormalities that may behave innocent at the beginning. And finally, ultrasound imaging was performed only through the anterior fontanel in most of the patients.
Conclusion
Brain anomalies represent a major issue in the health services due to their implication in the outcome of the patients. Cranial ultrasound is a non-invasive, very sensitive and safe tool for diagnosis and dynamic following of these patients.
The incidence of brain anomalies detected by ultrasound in present study was 0.506%. Intracranial haemorrhages and their possible consequences were the most common. Therefore, prematurity and other perinatal risk factors still present a major issue in Kosovo. Moreover, the rate of prenatal diagnosis was very low. Hence, familiarity with ultrasound features of brain anatomy, in particular during prenatal period, would facilitate an early diagnosis and proper management of major and rare congenital brain anomalies.