Introduction
In tropical countries, malaria is known as one of the most widespread infectious diseases with an estimated 212 million cases globally. Treatment of malaria has become more difficult when the drug resistance appears against parasites. Therefore, safe and effective new drugs are highly required. Because of simplicity, lower cost, low rate of serious complications, and greater tolerability, traditional medicine is an important source for new drugs.
Aim
To evaluate the anti-malaria and immune modulatory effects of Nigella sativa against Plasmodium berghei in vivo.
Materials and Methods
This study was conducted in the Department of Parasitology, the Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran during January 2014 to January 2015. N. sativa powder was macerated in methanol and filtered with Bokhner hopper, and solvent was separated in a rotary evaporator. In the result of toxicity test, the 450 mg/kg was determined as maximum dose with minimum toxicity. Antimalarial efficacy and interferon gamma (IFN-γ) and Interleukin 4 (IL-4) cytokines level were investigated in five groups of P. berghei-infected BALB/c mice. The percentage of parasitaemia and surveillance were also evaluated.
Results
The results of our study showed no toxicity, even at high concentration of herbal extract. A significant reduction in the percentage of parasitaemia was not observed in the treatment group. N. sativa-treated infected mice showed a significant increase in the IFN-γ but not IL-4 serum level, In addition, no higher surveillance was observed in the mice group treated with N. sativa compared to treated mice.
Conclusion
Hydroalcoholic extract of N. sativa has the weak efficiency against P. berghei. However, there is requirement to evaluate the immune mechanism and also find the major component of this herbal extract by further studies.
Introduction
Malaria is caused by members of genus Plasmodium. It is estimated that about 120 species are able to infect birds, reptiles, and mammals; however, only four of them induce the disease in humans, including Plasmodium vivax, P. malariae, P. ovale, and P. falciparum. These species are the reason of most of the deaths since they cause the most severe form of malaria [1]. In recent years, P. knowlesi, which normally infects the macaque monkeys, has been reported to infect humans and to be known as the fifth human malaria parasite [2]. Malaria, as the world’s most important tropical disease, is transmitted by an Anopheline mosquito vector. Malaria is responsible for killing a million people each year and infecting 212 million individuals worldwide [3]. However in Iran, it infected 3031 cases in 2010, and 1230 cases in 2014 [4-6].
Plasmodium spp., especially P. falciparum, is genetically complicated and has ability to cross-mate and produce mutant variants. Genetic mutant is responsible for pathogenicity and deficiency in immune responses. In these conditions, parasite leads to the emergence of resistance against almost all available anti-malarial drugs and helps to spread malaria to new areas and finally causes the recurrence of malaria in the regions where the disease has been eradicated. In such a situation, there is a need for developing new effective anti-malarial drugs [6].
Both humoral and cellular immune responses play a significant role in immunity. In humoral immunity, inflammation produces cytokines IL-4, IL-5, IL-10, and IL-12, which are stimulated by TH2 cells. In cellular immunity, the parasite enters the macrophages by binding collagen and laminin receptors on the surface of the macrophages, produces peneterating-enhancing factor and then duplicates. Finally, the infected macrophage produces IL-12, which increases the number of T helper 1 (Th1) cells. Th1 cells in turn produce interferon gamma (IFN-γ) [7].
Anti-malarial drug resistance is a major problem that often emerges when a parasite strain survives and/or multiply after exposing to different doses of a drug, equal to or higher than doses recommended with considering the tolerance of the subject [8,9].
Animal malaria species have widely been used for development of anti-malarial drugs. In 1948, Plasmodium berghei was introduced as a laboratory mouse strain. Due to high similarities with P. falciparum, as the causative agent of severe malaria in human, P. berghei has frequently been employed in research as a model for human malaria [10]. New drugs used against malaria should be inexpensive and routinely available to people living in malaria-epidemic areas.
Nigella sativa or black seed, known as Siah Daneh in Persian, mostly grows in the Southern Europe, North Africa, Middle East, and Western Asia. Traditionally, this plant has long been used as a natural medicine for the treatment of many acute and chronic diseases. Nigella sativa has various characteristics and features, including antioxidant [11], hepatoprotective [12] and respiratory [13] effects, as well as anti-inflamatory and analgestic [14], anticarcinogenic and antimutagenic [15], nephroprotective [16], immunomodulatory [17], antibacterial [18], antiulcer [19], and antiparasitic [20] activities. N. sativa has also been demonstrated to have some impacts on cardiovascular system and blood [21]. The seeds of this plant are composed of fixed and essential oils, proteins, alkaloids, and saponin. Evidence has shown that most of the biological activity of the seeds is due to thymoquinone, the major component of the essential oil; however, it is found in the fixed oil as well [22]. In spite of broad studies in the field of N. sativa seeds, few investigations have been conducted on antimalarial and immunomodulatory activity of N. sativa seeds against P. berghei ANKA strain in BALB/c mice. To achieve this goal, this study attempted to evaluate the antimalarial and immunomodulatory effects of N. sativa against P. berghei in vivo.
Materials and Methods
Sample Preparation
In this experimental in vivo study, six- to eight-week-old male BALB/c mice were obtained from the Razi Vaccine and Serum Research Institute (Karaj, Iran). The mice were allowed to acclimatize to housing condition for one week prior to the experiment and allowed food and water ad libitum. This study was conducted in the Department of Parasitology, the Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran, during January 2014 to January 2015 in accordance with the animal care and use protocol of Urmia University of Medical Sciences, Urmia, Iran (Ethical code: IR. UMSU.Rec. 1392.69).
The mice (n=50) were divided equally into five groups: Group 1, mice infected with P. berghei and treated with chloroquine (P.C.), group 2, mice infected with P. berghei and treated with N. sativa hydroalcoholic extract (P.E.), group 3, mice infected with P. berghei without any treatment (P.N.), group 4, no infection with P. berghei but treated with N. sativa hydroalcoholic extract (N.E.), and group 5, no infection with P. berghei and no treatment (N.N.). From each group, five mice were randomly selected for the measurement of IFN-γ and IL-4 levels, and another five mice were chosen for parasitemia determination and survival test.
Parasite Preparation
P. berghei ANKA 2.34 strain, donated by P.F. Billingsley, was kept in liquid nitrogen, melted in 37°C bath and injected peritoneally into mice. If the parasitemia was 25%, mice blood was diluted 100 times. Since the injected mice had parasitemia about 52%, bloods of all mice were diluted 200 times to achieve appropriate injection dose. Each mice from each group received 0.2 cc of diluted blood peritoneally [23].
Preparation of Hydroalcoholic Extract
N. sativa seeds of Iran origin were purchased from an herbal shop in Piranshahr, West Azerbaijan, Iran. Faculty of Agriculture of Urmia University verified the correctness of plants. The seeds were dried in shade at 35-40°C and crushed into coarse powder. To produce the hydroalcoholic extract, 200 gr of the seed powder was soaked into 800 mL of 70% ethanol. The solution was then shaken once every 24 hours for three days and then filtered through a whatman filter paper number one. Alcohol was removed from the extract solution by evaporation in the vacuum using a rotary device until a liquid gel was formed. This process was performed in order to determine pharmacological and toxicological dose (300, 450, and 600 mg/kg) of extract. Subsequently, each dose was injected into three mice. The general condition of mice was evaluated in 15, 30, 45, 60, 90, and 120 minutes after injection [23]. The movement of mice received the dose of 600 mg/kg decreased, and the mice became lethargic and sleepy, but in two other dose groups, no serious side effects were observed. Therefore, 450 mg/kg was determined as maximum dose with minimum toxicity.
Treatment of Mice
Treatment of mice started at the first day of parasite observation in the peripheral blood. Following the observation of P. berghei in blood of mice, treatment was performed with 0.2 cc of extract or 20 mg/kg of chloroquine for four consecutive days intraperitoneally [23].
Parasitemia Determination
Three days after treatment, blood smear (tail blood) was prepared and stained with Giemsa, and the percentage of infected erythrocytes was determined. For this purpose, 1000 RBC were counted, and the percentage of parasitemia was determined.
Measurement of IFN-γ and IL-4 Levels
Levels of IFN-γ and IL-4 were measured in the sera of five mice from each group by using ELISA (mouse IFN-gamma platinum ELISA, BMS 606 and mouse IL-4 platinum ELISA, BMS613, eBioscience, USA) according to the instruction provided by the manufacturer.
The Survival Rate of Mice
Following the observation of P. berghei in the blood of five mice in groups P.C., P.E., and P.N. (P.C.: mice infected with P. berghei and treated with chloroquine, P.E.: mice infected with P. berghei and treated with N. sativa hydroalcoholic extract, P.N.: mice infected with P. berghei without any treatment), the survival rate of mice was monitored for 20 days.
Statistical Analysis
Data analysis was carried out using Kaplan Meier, ANOVA, and t-test. Significant level was determined statistically as p<0.05.
Results
The serum IFN-γ level in different groups was evaluated, and its result is exhibited in [Table/Fig-1]. The statistical analysis in this section was conducted using ANOVA test. The mean secretion of IFN-γ in P.E. group was significantly more than N.N. group (p=0.008), and that of N.E. group was significantly higher than both P.C. and N.N. groups p=0.05 and p=0.003, respectively. The mean secretion difference between P.N. and N.N. groups were also significant (p=0.021). In spite of differences in IFN-γ rate between P.E. and P.C. groups, it was not statistically significant (p=0.89).
Mean of INF-γ secretion in studied mice groups.
| Groups | Number of mice | Mean | Std. Deviation | Std. Error | Min | Max |
|---|
| P.C | 5 | 20.66 | 13.8132 | 6.1734 | 7.64 | 44.19 |
| P.N | 5 | 28.13 | 10.2943 | 4.6083 | 13.99 | 38.63 |
| N.E | 5 | 39.10 | 12.6743 | 5.6674 | 21.94 | 56.11 |
| P.E | 5 | 31.76 | 10.4687 | 4.6749 | 21.94 | 44.19 |
| N.N | 5 | 13.57 | 4.9316 | 2.2052 | 8.52 | 21.49 |
The serum rate of IL-4 was elevated in different groups, as illustrated in [Table/Fig-2]. Results showed a significant difference between P.N. and P.C. groups (p=0.024), but no significant difference was found in the secretion mean of IL-4.
Mean of IL-4 secretion in studied mice groups.
| Groups | Number of mice | Mean | Std. Deviation | Std. Error | Min | Max |
|---|
| P.C | 5 | 43.4920 | 13.16312 | 5.16606 | 26.22 | 61.02 |
| P.N | 5 | 74.4756 | 23.29319 | 29.0000 | 57.16 | 88.61 |
| N.E | 5 | 60.5074 | 17.223601 | 7.70818 | 44.27 | 88.09 |
| P.E | 5 | 69.7868 | 31.93453 | 14.28156 | 40.40 | 98.40 |
| N.N | - | - | - | - | - | - |
Parasitemia and Survival Time
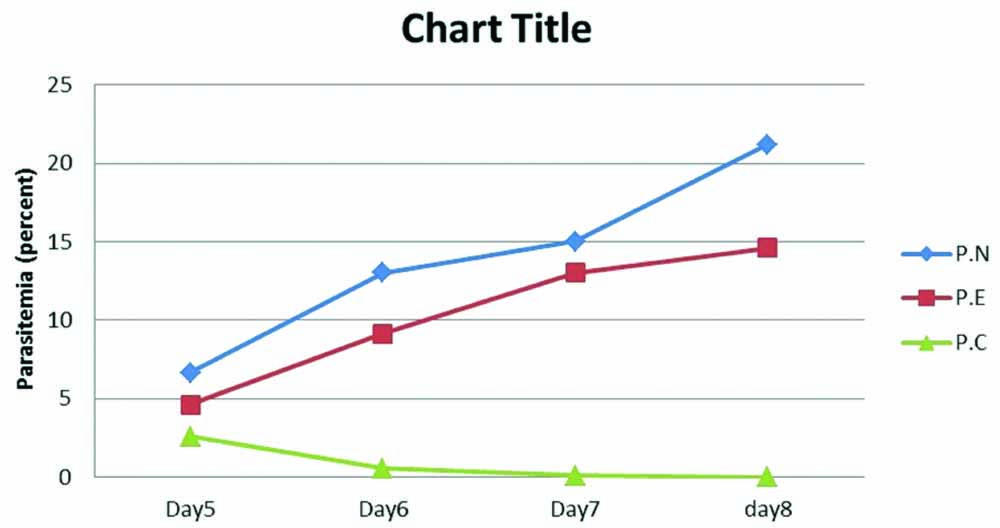
Parasitemia rate in different groups were assessed, and the mean percentage of parasites in 5, 6, 7, and 8 days is shown in [Table/Fig-3,4]. Results demonstrated that parasitemia decreased in P.C. group but increased in both P.N. and P.E. groups. Also, in P.E. group, the parasitemia rate in day four slightly increased compared to P.N. group.
Mean of parasitemia in infected groups with P. berghei.
| Day 5 | Day 6 | Day 7 | Day 8 |
|---|
| P.N | 2.2 | 5.1 | 10 | 20 |
| 11 | 12 | 15 | 21 |
| 12 | 21 | 21 | 29 |
| 7 | 22 | 20 | 24 |
| 1 | 5 | 9 | 12 |
| Average | 6.64 | 13.02 | 15 | 21.2 |
| P.E | 6.2 | 9 | 15.6 | 17.2 |
| 4.4 | 7.4 | 17.6 | 17.8 |
| 6.2 | 10 | 15.4 | 18.4 |
| 1.4 | 3.4 | 11.2 | 11.4 |
| 5 | 16 | 5.3 | 8.3 |
| Average | 4.64 | 9.16 | 13.02 | 14.62 |
| P.C | 2.1 | 0.33 | 0 | 0 |
| 2.8 | 1.33 | 0.28 | 0 |
| 4.2 | 0.21 | 0 | 0 |
| 2.2 | 0.33 | 0.21 | 0 |
| 2.6 | 0.69 | 0 | 0 |
| Average | 2.58 | 0.578 | 0.098 | 0 |
Rate of parasitemia in mice infected with P. berghei. Statistical analysis was done with the ANOVA test.
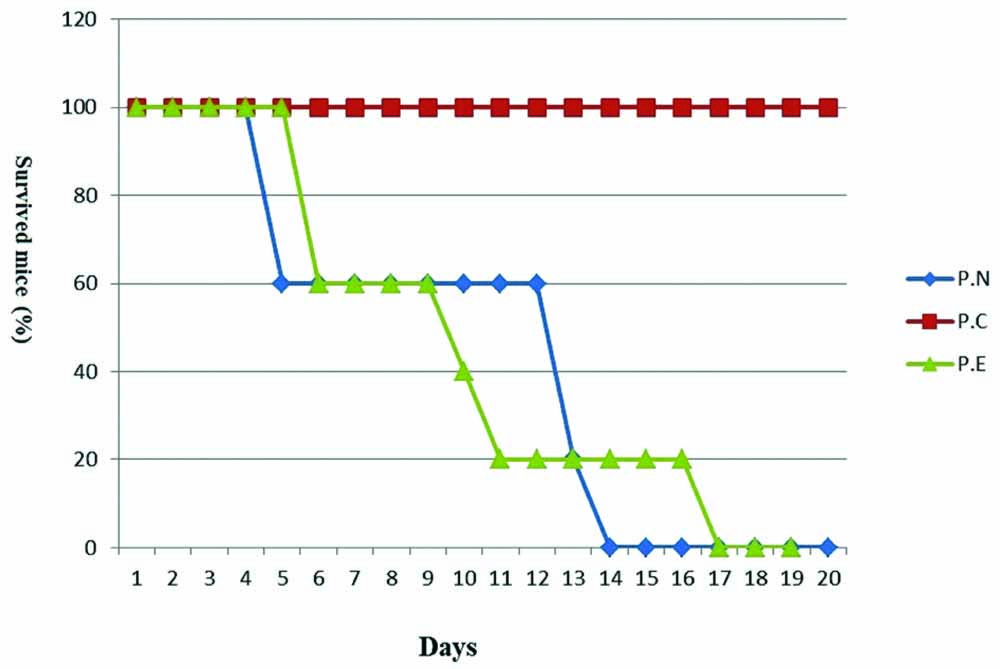
The highest and the lowest survival times were observed in P.C. and control groups, respectively. The last mouse survived in P.N. group died in 14th day, but in P.E. group, the mouse died in 17th day [Table/Fig-5].
Survival rates of mice in P.N, P.C, and P.E. Statiscal analysis was done with Kaplan Meier test.
The results from parasitemia and survival time in uninfected groups (N.E. and N.N.) are not mentioned in [Table/Fig-4].
Discussion
There are various anti-malarial drugs available in the market such as quinine antifolate, combination drugs, antibiotics, artemisinin compounds, and miscellaneous compounds. The greatest challenge against malaria control program emerges when the parasite shows resistant against antimalarial drugs. Our results suggested that due to the anti-plasmoidal activity, N. sativa ethanolic extract is able to partly kill or prevent duplication of the parasite. It means that this herbal drug is capable of suppressing the parasite and can lengthen the lifespan of an infected mouse to some extent. Previous studies have also found similar results regarding parasites [24], especially P. bergei [23]. Abdulelah H et al., have examined different doses of ethanol aqueous and chloroform extracts of N. sativa and concluded that ethanol and chloroform extracts at 100 μL/kg have the best result in suppression and survival time [23]. Similarly, Emeka PM et al., have revealed that N. sativa extract in dose of 10 mg/kg can cause suppression and increases survival time [25]. Their results are partly in agreement with our outcome in terms of increasing the survival time and preventing the duplication of parasites; however, different injection place causes difference in ideal dose. Production of IFN-γ in response to N. sativa extract has been mentioned in other studies. In almost all pathogens, responses of immune system starts with innate immune reactions, which initiates with the secretion of TNF-α, activation of acquired immune system induced with Th1 and Th2 cells, and the secretion of IFN-γ, IL-2, IL-6, IL-8, and IL-4, IL-5, IL-9, and IL-13.
Herbal drugs show their immunomodulatory actions with secreting particular cytokines [26]. High secretion of IFN-γ is a favorable outcome in almost all malaria animal models and play a key role in human defense system against plasmodium parasite by producing high levels of nitric oxide [27,28]. In the current work, the extract of N. sativa increased the production of IFN-γ, but not as much as P.C. group. Walther M et al., have demonstrated that the excessive levels of proinflammatory cytokines such as IFN-γ may produce cerebral dysfunction in malaria-infected individuals [29]. However, the limited increase in IFN-γ production, as mentioned in the present study, not only restricted malaria infection but also controlled the cerebral disease caused by plasmodium parasite.
IL-4 evaluation exhibited that the effect of N. sativa extract between different groups was not statistically significant. This result was in accordance with other studies who evaluated the impact of N. sativa extract on human lymphocyte. It was found that N. sativa extract significantly increased the levels of IL-1 and IL-3, but it did not have any significant effect on IL-2 and IL-4 [30].
In this study, the same as Lalloo DG et al., study, we used chloroquine to evaluate the activity of parasite and choice drug for the treatment of non-falciparum infections and non-severe falciparum infections, which are acquired in the areas of chloroquine sensitivity [31].
Limitation
More evaluation is needed regarding the separation of the individual compounds presented in N. sativa extract to achieve more effective material. These compounds could contribute to develop new drugs against Plasmodium species in the drug resistance situation.
Conclusion
We expected that the hydroalcoholic extract of Nigella sativa has the same efficiency as chloroquine or even better against Plasmodium berghei, but the extract showed a very weak effect. The extract increased the survival time by three days in P.E. group compared to P.N. group, but comparison of P.E. and P.C. groups indicated very weak efficacy of the extract. However, more investigations are needed for the fractionation of N. sativa in order to find the active principles responsible for its anti-malarial activity.
[1]. Gregson A, Plowe CV, Mechanisms of resistance of malaria parasites to antifolatesPharmacological Reviews 2005 57(1):117-45.10.1124/pr.57.1.415734729 [Google Scholar] [CrossRef] [PubMed]
[2]. White N J, Plasmodium Knowlesi: The fifth human Malaria parasiteClinical Infectious Disease 2008 46:172-73.10.1086/52488918171246 [Google Scholar] [CrossRef] [PubMed]
[3]. http://www.who.int/features/factfiles/malaria/en/ [Google Scholar]
[4]. World Health Organization. World health statistics 2016: monitoring health for the SDGs sustainable development goals: World Health Organization; 2016 [Google Scholar]
[5]. Sheikhzadeh K, Haghdoost AA, Bahrampour A, Zolala F, Raeisi A, Assessment of the impact of the malaria elimination programme on the burden of disease morbidity in endemic areas of IranMalaria Journal 2016 15(1):20910.1186/s12936-016-1267-927074734 [Google Scholar] [CrossRef] [PubMed]
[6]. Salmanzadeh S, Foroutan-Rad M, Khademvatan S, Moogahi S, Bigdeli S, Significant decline of malaria incidence in southwest of Iran (2001-2014)Journal of Tropical Medicine 2015 2015:52376710.1155/2015/52376726649056 [Google Scholar] [CrossRef] [PubMed]
[7]. Suzuki Y, Orellana MA, Schreiber RD, Remington JS, Interferon-gamma: the major mediator of resistance against Toxoplasma gondiiScience 1988 240(4851):516-18.10.1126/science.31288693128869 [Google Scholar] [CrossRef] [PubMed]
[8]. Bruce-Chwatt LJ, Black RH, Canfield CJ, Clyde DF, Peters W, Chemotherapy of malaria 1986 rev. 2nd edGenevaWorld Health Organization [Google Scholar]
[9]. Lobel H, Campbell C, Malaria prophylaxis and distribution of drug resistanceClinics in tropical medicine and communicable diseases 1986 1(1):225-42. [Google Scholar]
[10]. Miandoabi T, Hazrati Tappeh Kh, Nahravanian H, Kazemi SM, Mohammadzadeh H, Hanifian H, Pharmacochemistry of Iranian flora Artemisia oliveriana and its antimalarial effect on Plasmodium berghei in vivoAdvanced Studies in Biology 2017 9(1):43-51.10.12988/asb.2017.61141 [Google Scholar] [CrossRef]
[11]. Saxena S, Rathore S, Diwakar Y, Kakani R, Kant K, Dubey P, Genetic diversity in fatty acid composition and antioxidant capacity of Nigella sativa L. genotypesLWT-Food Science and Technology 2017 78:198-207.10.1016/j.lwt.2016.12.033 [Google Scholar] [CrossRef]
[12]. Ali H, Korshom MA, Mandour AA, El-Bessoumy AA, El-Sayed YS, Hepatoprotective efficacy of Nigella sativa seeds dietary supplementation against lead acetate-induced oxidative damage in rabbit-Purification and characterization of glutathione peroxidaseBiomedicine & Pharmacotherapy 2017 89:711-18.10.1016/j.biopha.2017.02.04428273633 [Google Scholar] [CrossRef] [PubMed]
[13]. Koshak A, Wei L, Koshak E, Wali S, Alamoudi O, Demerdash A, Nigella sativa supplementation improves asthma control nd biomarkers: A randomized, double-blind, placebo-controlled trialPhytotherapy Research 2017 31(3):403-09.10.1002/ptr.576128093815 [Google Scholar] [CrossRef] [PubMed]
[14]. Chehl N, Chipitsyna G, Gong Q, Yeo CJ, Arafat HA, Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cellsHPB 2009 11(5):373-81.10.1111/j.1477-2574.2009.00059.x19768141 [Google Scholar] [CrossRef] [PubMed]
[15]. Majdalawieh AF, Fayyad MW, Nasrallah GK, Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativaCritical Reviews in Food Science and Nutrition 2017 Dec 12 57(18):3911-28.10.1080/10408398.2016.127797128140613 [Google Scholar] [CrossRef] [PubMed]
[16]. Casanova AG, Vicente-Vicente L, Hernández-Sánchez MT, Pescador M, Prieto M, Martínez-Salgado C, Key role of oxidative stress in animal models of aminoglycoside nephrotoxicity revealed by a systematic analysis of the antioxidant-to-nephroprotective correlationToxicology 2017 385:10-17.10.1016/j.tox.2017.04.01528472626 [Google Scholar] [CrossRef] [PubMed]
[17]. Oyero OG, Onifade AA, Baba M, Immunomodulatory potential of herbal formulations containing seeds of Nigella sativa LinnAfrican Journal of Biomedical Research 2017 20(2):217-21. [Google Scholar]
[18]. Bakal SN, Bereswill S, Heimesaat MM, Finding novel antibiotic substances from medicinal plants-Antimicrobial properties of Nigella sativa directed against multidrug resistant bacteriaEuropean Journal of Microbiology and Immunology 2017 7(1):92-98.10.1556/1886.2017.0000128386474 [Google Scholar] [CrossRef] [PubMed]
[19]. Manjegowda SB, Rajagopal HM, Dharmesh SM, Polysaccharide of Black cumin (Nigella sativa) modulates molecular signaling cascade of gastric ulcer pathogenesisInternational Journal of Biological Macromolecules 2017 101:823-36.10.1016/j.ijbiomac.2017.03.09328322956 [Google Scholar] [CrossRef] [PubMed]
[20]. Ullah R, Rehman A, Zafeer MF, Rehman L, Khan YA, Khan MH, Anthelmintic Potential of Thymoquinone and Curcumin on Fasciola giganticaPloS one 2017 12(2):e017126710.1371/journal.pone.017126728152102 [Google Scholar] [CrossRef] [PubMed]
[21]. Norouzi F, Abareshi A, Asgharzadeh F, Beheshti F, Hosseini M, Farzadnia M, The effect of Nigella sativa on inflammation-induced myocardial fibrosis in male ratsResearch in Pharmaceutical Sciences 2017 12(1):7410.4103/1735-5362.19905028255317 [Google Scholar] [CrossRef] [PubMed]
[22]. Ali B, Blunden G, Pharmacological and toxicological properties of Nigella sativaPhytotherapy Research 2003 17(4):299-305.10.1002/ptr.130912722128 [Google Scholar] [CrossRef] [PubMed]
[23]. Abdulelah H, Zainal-Abidin B, In vivo anti-malarial tests of Nigella sativa (Black Seed) different extractsAm J Pharmacol Toxicol 2007 2(2):46-50.10.3844/ajptsp.2007.46.50 [Google Scholar] [CrossRef]
[24]. Shenawy E, Nahla S, Soliman MF, Reyad SI, The effect of antioxidant properties of aqueous garlic extract and Nigella sativa as anti-schistosomiasis agents in miceRevista do Instituto de Medicina Tropical de São Paulo 2008 50(1):29-36.10.1590/S0036-4665200800010000718327484 [Google Scholar] [CrossRef] [PubMed]
[25]. Emeka PM, Badger-Emeka LI, Eneh CM, Khan TM, Dietary supplementation of chloroquine with Nigella sativa seed and oil extracts in the treatment of malaria induced in mice with Plasmodium bergheiPharmacognosy magazine 2014 10(Suppl 2):S35710.4103/0973-1296.13328224991115 [Google Scholar] [CrossRef] [PubMed]
[26]. Sedegah M, Finkelman F, Hoffman SL, Interleukin 12 induction of interferon gamma-dependent protection against malariaProceedings of the National Academy of Sciences 1994 91(22):10700-02.10.1073/pnas.91.22.107007938013 [Google Scholar] [CrossRef] [PubMed]
[27]. Stevenson MM, Tam MF, Wolf SF, Sher A, IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanismThe Journal of Immunology 1995 155(5):2545-56. [Google Scholar]
[28]. Jacobs P, Radzioch D, Stevenson MM, In vivo regulation of nitric oxide production by tumor necrosis factor alpha and gamma interferon, but not by interleukin-4, during blood stage malaria in miceInfection and Immunity 1996 64(1):44-49. [Google Scholar]
[29]. Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, Distinct roles for FOXP3+ and FOXP3- CD4+ T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malariaPLoS Pathogens 2009 5(4):e100036410.1371/journal.ppat.100036419343213 [Google Scholar] [CrossRef] [PubMed]
[30]. Haq A, Abdullatif M, Lobo PI, Khabar KS, Sheth KV, al-Sedairy ST, Nigella sativa: Effect on human lymphocytes and polymorphonuclear leukocyte phagocytic activityImmunopharmacology 1995 30(2):147-55.10.1016/0162-3109(95)00016-M [Google Scholar] [CrossRef]
[31]. Lalloo DG, Shingadia D, Pasvol G, Chiodini PL, Whitty CJ, Beeching NJ, UK malaria treatment guidelinesJournal of Infection 2007 54(2):111-21.10.1016/j.jinf.2006.12.00317215045 [Google Scholar] [CrossRef] [PubMed]