Case Report
A 20-year-old female presented to the emergency department with chief complaints of fever for two days and altered behaviour from one day, without any episode of seizure. No similar episodes occurred in past neither was there a family history.
On physical examination, her Glasgow Coma Scale (GCS) was E2V2M5, she was febrile (102°F), pulse rate was 90/minute regular and Blood Pressure (BP) was 100/70 mmHg. Neck rigidity and Kernig’s sign were negative. On palpation of abdomen, there was splenomegaly 5 cm below left coastal margin. Fundus examination was normal. All other systemic examinations were within normal limit. Patient was suspected as a case of inflammatory brain disease like acute viral encephalitis, complicated malaria, pyogenic meningitis, septic encephalopathy, aseptic meningitis like leptospirosis, scrub typhus, dengue fever.
Initial blood tests revealed haemoglobin (Hb) 9.22 gm/dL, Total Leukocyte Count (TLC) 3100 cells/mm3, neutrophils 75%, lymphocytes 24% and platelet count of 70,000 cells/mm3. Serum sodium (Na+) 144 mmol/L, potassium (K+) 4.3 mmol/L, blood urea 54 mg/dL and serum creatinine 0.86 mg/dL. Serum bilirubin (total) was 4.73 mg/dL and direct fraction was 1.3 mg/dL. Serum Glutamic Oxaloacetic Transaminase (SGOT) was 37 IU/L, Serum Glutamic Pyruvic Transaminase (SGPT) 35 IU/L, Serum Alkaline Phosphate (SALP) 341 IU/L. serum total protein (6.2 mg/dL) and albumin was 3.7 mg/dL while prothrombin time (PT)/International Normalised Ratio (INR) was 25.9 seconds/2.45. As patient had deranged liver function viral markers hepatitis B virus surface antigen (HBsAg) and Hepatitis C Virus (HCV) tests were done and found to be negative.
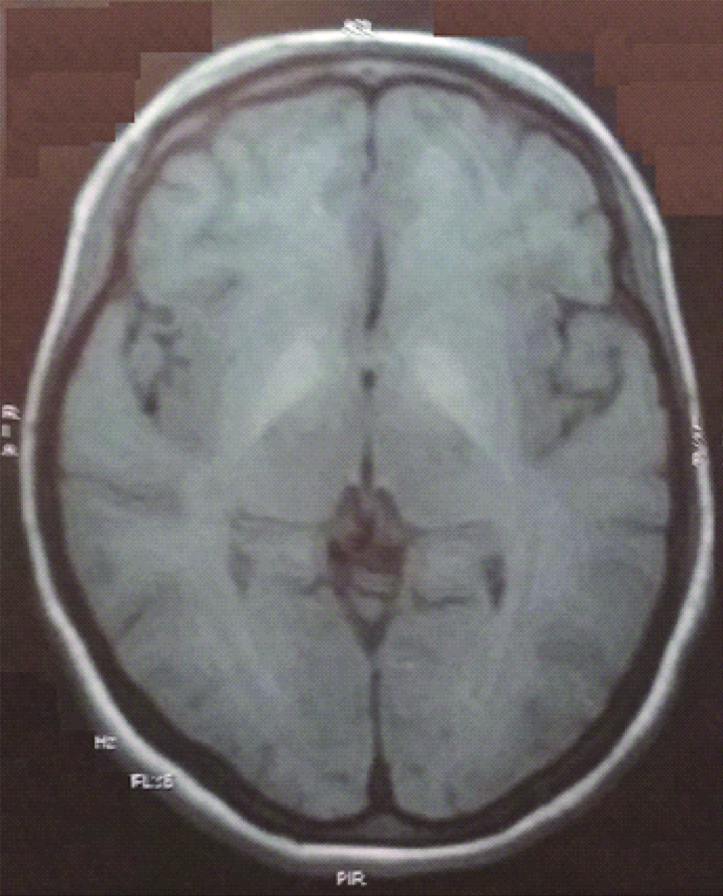
Non-Contrast Computed Tomography (NCCT) brain showed no abnormality. Cerebrospinal Fluid (CSF) examination showed TLC less than 5 cells/mm3, all lymphocytes with protein 27 mg/dL and sugar 108 mg/dL with corresponding blood sugar level of 138 mg/ dL, which was normal. Magnetic Resonance Imaging (MRI) showed T1 hyperintensity in bilateral putamen area which was suggestive of viral encephalitis [Table/Fig-1]. However, CSF viral markers like Japanese encephalitis, entero virus, herpes simplex virus 1, Varicella Zoster Virus (VZV) were negative. Blood smear and rapid card test for malaria parasite were both negative. Serum viral markers for NS1 antigen, IgM dengue, IgM leptospira and IgM scrub typhus were negative.
T1W MRI of the patient showing hyperintensity in bilateral basal ganglia.
Patient was managed with intravenous antibiotics (ceftriaxone), antimalarial (artesunate), antiviral (acyclovir) and steroid (dexamethasone) while we waited for confirmation of diagnosis. Patient became conscious, oriented (GCS E4V5M6) and afebrile on 3rd day of admission. On day 5th of admission, Hb was 9.8 gm/dL, TLC 2,290 cells/mm3, polymorphs 54%, lymphocytes 28%, eosinophils 10%, Mean Cell Volume (MCV) 85.9 fl., Red Cell Distribution Width (RDW) 13.9%, and platelet count 26,900 cells/mm3.
For anaemia we looked for concomitant causes like deficiency of vitamin B12, folic acid, iron status but their serum levels were normal and stool for occult blood was negative. On day 8th of admission, blood count was repeated which showed further decrease in values of blood cells with Hb 9.3 gm/dL, TLC 1,350 cells/mm3, polymorphs 72%, lymphocytes 14%, eosinophils 06% and platelet count 46,500 cells/mm3. On 10th day, patient’s Hb was 8.6 gm/dL, TLC 700 cells/mm3, polymorphs 60%, lymphocytes 25% and platelet count 43000 cells/mm3 [Table/Fig-2].
Showing changing laboratory parameters on successive days.
| Lab parameters | DAY 1 | DAY 5 | DAY 8 | DAY 10 | DAY 18 | One month after discharge |
|---|
| Hb (gm/dL) | 9.22 | 9.8 | 9.3 | 8.6 | 9 | 10.2 |
| TLC (cells/mm3) | 3100 | 2290 | 1350 | 700 | 1500 | 6200 |
| DLC (%) | N 75, L 24 | N 54, L 28 | N 72, L 14 | N 60, L 25 | N 66, L 26 | N67, L28 |
| Platelets (cells/mm3) | 70000 | 26900 | 46500 | 43000 | 77000 | 110000 |
| S. bilirubin (mg/dL) | 4.73 | | | | | 2.1 |
| SGOT (IU/L) | 37 | | | | | 26 |
| SGPT (IU/L) | 35 | | | | | 30 |
| SALP (IU/L) | 341 | | | | | 280 |
Hb: Haemoglobin; TLC: Total leukocyte count; DLC: Differential leukocyte count; SGOT: Serum glutamic oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase; SALP: Serum alkaline phosphate
Although patient was apparently normal but with further decline in cell count, bone marrow examination was done for suspicion of leukaemia leading to leukaemic encephalopathy. However bone marrow aspiration/cytology was normocellular for age, megakaryocytes were increased, myeloid: erythroid ratio was 9:1. Granulocytic precursors (85%) showed maturation with increased myelocytes and metamyelocytes. Erythroid precursors (06%) showed increased early giant erythroid precursors with intranuclear inclusions. There was erythroid suppression and myeloid maturation with left shift. In bone marrow cytology, inclusion bodies in erythroid precursor were suggestive of some viral infection causing transient bone marrow suppression, so serum for antibody against epsteinbarr virus, parvo virus, cytomegalo irus and human herpes virus 6 were tested, which could explain the cause of pancytopenia followng encephalitic syndrome. Serum anti-parvo B-19 virus Ig M antibody as well as HPV B-19 DNA was found positive.
On day 18th after admission, there was slight improvement in her cell counts with Hb 9.0 gm/dL, TLC 1500 cells/mm3, polymorphs 66%, lymphocytes 26% and Platelet count 77,000 cells/mm3. Clinically spleen did not regress. Patient’s history was again reviewed and patient narrated that she had undergone ascitic tap 2 years ago but no record was found or further evaluation was done as patient remained asymptomatic. To rule out systemic lupus erythematosus leading to vasculitis with serositis and thrombocytopenia in a female, ANA was done which showed positivity of 2+. Ultrasonography (USG) abdomen was done which showed coarse echo-texture of liver with portal vein thrombosis with spleenomegaly of size 19.5 cm without free fluid in peritoneum, suggestive of chronic liver disease.
Since HbSAg and HCV test were negative and she was young, hence Wilson’s disease was suspected where MRI finding had shown T1 hyperintensity in bilateral putamen area [Table/Fig-1]. Kayserfleischer (KF) ring was absent but 24 hours urinary copper was 350 ug (normal range 15-70 ug/24 hours) and serum ceruloplasmin level was 0.23 g/L (normal range 0.20-0.60 g/L), which was suggestive of Wilson’s disease. No similar history was there in the family. Upper GI endoscopy showed antral and duodenal polyps but did not show varices.
For Wilson’s disease, zinc acetate was given for controlling copper level. Patient is continuing on above medication for the past six months and is symptomatically better.
Discussion
Parvo virus mainly affects animals but in 1975 first time Cossart et al., discovered infections in human [1].
While in 1980, first patient with parvo virus infection presenting as febrile illness was discovered by Shneerson JM et al., [2]. Various clinical manifestations caused by B19 in healthy host are Erythema Infectiosum (EI) [3], arthropathy, non-immune hydropsfetalis, thrombocytopenia, congenital anaemia, hepatitis, myocarditis and neurologic diseases, while in immunodeficient hosts chronic pure red cell aplasia and in patients with raised red cell turnover, transient aplastic crisis [4]. Among all these, neurologic manifestations are increasing day by day [5,6]. Wilson disease is an inherited disease of copper accumulation leading to copper toxicity in brain and liver. It predominantly presents as episodes of acute hepatitis, extra-pyramidal features, or neuropsychiatric manifestations [7].
This case was of Wilson’s disease, aggravated by recent HPV B19 infection. Initial presentation was similar to acute encephalitis syndrome, which may be due to HPV B19 infection. Common manifestations of HPV B19 infection are mild nonspecific prodromal illness due to viremia consisting of fever (in 15-30% of patients), headache, malaise, nausea, myalgia and rhinorrhea; after the initial infection of 5-7 days. Bright red macular exanthema on cheeks and diffuse maculopapular rash can appear one week later. Manifestations are of varied type and the classic “slapped cheek” rash commonly found in children. However, presentation as encephalitis syndrome is very rare [8]. Patient may be a case of hepatic encephalopathy due to deranged liver function. Wilson’s disease presents with acute liver failure in 5% of patients [9]. HPV B19 may present as fulminant hepatitis [9].
Mechanism of liver injury by B19 is not known and may be due to direct viral invasion or an indirect consequence of the immune response directed against the parvo virus B19 [10]. Autoimmune disorders like systemic lupus erythematous, rheumatoid arthritis, vasculitides, are associated with HPV B19 infection. Antinuclear Antibody (ANA) was also positive in our patient showing autoimmune association of the patient [11]. Bone marrow examination was suggestive of erythroid suppression and myeloid maturation with left shift. HPV B19 significantly have aplastic marrow effects in immunodeficient individuals which must be differentiated with acquired aplastic anaemia [12]. T1W hyperintensity in bilateral putamen may be due to varied causes like calcium and phosphate abnormalities, idiopathic calcification, Acquired (non-wilsonian) hepatocerebral degeneration, carbon monoxide poisoning, non-ketotic Hyperglycemic hemichorea, Japanese encephalitis, hamartoma in neurofibromatosis type 1 [13].
Conclusion
HPV B19 infection is a rare cause of acute encephalitis syndrome with hepatitis and pancytopenia. Wilson’s disease should be suspected in a young adult when the features are suggestive of chronic liver disease with portal hypertension. Pancytopenia due to HPV B19 can be reversible as it is a self limiting disease but in patient’s of Wilson’s disease who has landed in decompensated liver disease, this can be due to hypersplenism.
Hb: Haemoglobin; TLC: Total leukocyte count; DLC: Differential leukocyte count; SGOT: Serum glutamic oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase; SALP: Serum alkaline phosphate
[1]. Young NS, Brown KE, Parvovirus B19N Engl J Med 2004 350(6):586-97.10.1056/NEJMra03084014762186 [Google Scholar] [CrossRef] [PubMed]
[2]. Shneerson JM, Mortimer PP, Vandervelde EM, Febrile illness due to a parvovirusBr Med J 1980 280(6231):158010.1136/bmj.280.6231.15807427176 [Google Scholar] [CrossRef] [PubMed]
[3]. Anderson LJ, Role of parvovirus B19 in human diseasePediatr Infect Dis J 1987 6:71110.1097/00006454-198708000-000032823211 [Google Scholar] [CrossRef] [PubMed]
[4]. Heegaard ED, Brown KE, Human parvovirus B19Clin Microbiol Rev 2002 15(3):485-505.10.1128/CMR.15.3.485-505.200212097253 [Google Scholar] [CrossRef] [PubMed]
[5]. Douvoyianni M, Litman N, Goldman DL, Neurologic manifestations associated with parvovirus B19 infectionClin Infect Dis 2009 48(12):1713-23.10.1086/59904219441978 [Google Scholar] [CrossRef] [PubMed]
[6]. Barah F, Whiteside S, Batista S, Morris J, Neurological aspects of human parvovirus B19 infection: A systematic reviewRev Med Virol 2014 24(3):154-68.10.1002/rmv.178224459081 [Google Scholar] [CrossRef] [PubMed]
[7]. Rodriguez-Castro KI, Hevia-Urrutia FJ, Sturniolo GC, Wilson’s disease: A review of what we have learnedWorld J Hepatol 2015 7(29):285910.4254/wjh.v7.i29.285926692151 [Google Scholar] [CrossRef] [PubMed]
[8]. Brown KE, Anderson SM, Young NS, Erythrocyte P antigen: Cellular receptor for B19 parvovirusScience 1993 262(5130):114-17.10.1126/science.82111178211117 [Google Scholar] [CrossRef] [PubMed]
[9]. Hevia FJ, Miranda M, The special problem of wilson’s disease in costa rica - an unexpected high prevalenceGastroenterol Int 1989 1:228 [Google Scholar]
[10]. Hatakka A, Klein J, He R, Piper J, Tam E, Walkty A, Acute hepatitis as a manifestation of parvovirus B19 infectionJ Clin Microbiol 2011 2011:0057510.1128/JCM.00575-1121734024 [Google Scholar] [CrossRef] [PubMed]
[11]. Lunardi C, Tinazzi E, Bason C, Dolcino M, Corrocher R, Puccetti A, Human parvovirus B19 infection and autoimmunityAutoimmun Rev 2008 8(2):116-20.10.1016/j.autrev.2008.07.00518700174 [Google Scholar] [CrossRef] [PubMed]
[12]. Serjeant GR, Mason K, Topley JM, Serjeant BE, Pattison JR, Jones SE, Mohamed R, Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agentThe Lancet 1981 318(8247):595-97.10.1016/S0140-6736(81)92739-2 [Google Scholar] [CrossRef]
[13]. Zaitout Z, Romanowski C, Karunasaagarar K, Connolly D, Batty R, A review of pathologies associated with high T1W signal intensity in the basal ganglia on Magnetic Resonance ImagingPol J Radiol 2014 79:12610.12659/PJR.89004324900164 [Google Scholar] [CrossRef] [PubMed]