Introduction
Adenocarcinoma of the prostate may be clinically suspected based on elevated serum PSA (Prostate Specific Antigen) and/or abnormal digital rectal examination. Serum PSA has been used in prostatic carcinoma screening and for diagnostic, therapeutic and prognostic purposes.
Aim
To evaluate the role of serum PSA to differentiate between benign and malignant pathology of prostate diagnosed by Transrectal Ultrasound (TRUS) guided prostatic core biopsy with special emphasis on its correlation with risk stratification based on Gleason score and Grade grouping system.
Materials and Methods
Total 45 cases of Prostatic core biopsy specimens of the patients where serum PSA value was known were included in this cross-sectional observational study. Biopsy specimens were processed for routine Haematoxylin-Eosin stain and diagnosed as benign or malignant by histopathological examination along with Gleason score and Grade-Grouping was done. The comparison of PSA value between benign and malignant cases along with its significance of association with higher Grade-Grouping was statistically judged by unpaired t-test.
Results
Out of total 45 cases, 23 (51.11%) cases showed presence of adenocarcinoma and rest 22 cases were of benign pathology. Mean PSA value (ng/mL) was significantly higher in malignant cases i.e., 58.47 (±22.191) compared to benign cases i.e., 3.45 (±0.987). However, in malignant cases which have been categorised as low to intermediate risk group (Gleason score <8) and high risk group (Gleason score ≥8), the mean PSA values (ng/mL) were 58.75 and 58.18 respectively and they were not so statistically significant (p-value=0.95) to differentiate between these two groups.
Conclusion
Estimation of serum PSA has got definite significance in differentiating benign cases from malignant cases in prostatic core biopsy specimens (TRUS guided). But in cases of prostate adenocarcinoma while considering the disease stage based on Gleason Score and Grade-grouping, the serum PSA value has got no statistically significant role.
Introduction
Worldwide clinically detected prostate cancer is the third most common malignancy in men with an estimated about 1.2 million new cases in 2018 [1]. The highest incidences are in North America, Europe, The Caribbean and Brazil; whereas it is relatively less in Asia, Middle East and Africa. Most of the prostate cancers are detected in men aged >60 years. As per WHO (World Health Organization) Classification of Tumours of the Urinary System and Male Genital Organs, 4thed, only 1% of prostate cancers are clinically detected in male aged less than 50 years [2].
Most of the prostatic adenocarcinomas (85-90%) are multifocal with an average of 2-3 separate tumours per gland. Most of the prostate adenocarcinomas (75-80%) are located in the posterior/posterolateral peripheral zone [3].
Adenocarcinoma of the prostate may be clinically suspected based on elevated serum PSA and/or abnormal Digital Rectal Examination (DRE). Serum PSA level generally correlate with the risk of prostate cancer and hence serum PSA has been used in prostatic carcinoma screening and for diagnostic, therapeutic and prognostic purposes [2,4]. However, as a screening test for prostate cancer, the use of single serum PSA estimation remains controversial in that it lacks both sensitivity and specificity [5]. In most laboratories, a serum level of 4 ng/mL is considered as the cut-off between normal and abnormal but many men with PSA levels below this cut off point may harbour cancer cells in their prostate specially in cases of organ-confined prostate cancer [5,6]. Generally, a biopsy is recommended if the DRE or PSA is abnormal. The PSA level establishes the likelihood that a man will harbour prostate cancer if he undergoes a prostate biopsy. Men with an abnormal PSA and negative biopsy are usually advised to undergo a repeat biopsy [6].
Fine needle aspiration cytology of the prostate was widely used for diagnosing prostate cancer before core needle technique was developed [7].
However, currently TRUS is the first modality of choice to image and biopsy in case of suspected prostatic pathology [8,9]. Current standard of care is to obtain systematic prostate 18 gauge core biopsies guided by transrectal ultrasound from suspected areas as identified by digital rectal examination and imaging [8,10]. Toi A et al., reviewed 7426 prostate biopsies and found that the presence of a sonographic lesion significantly increased the likelihood of prostate cancer detection [11].
In present study, it was observed that each specimen of prostatic core biopsy done by transrectal ultra sound guided method and evaluated the percentage of positivity of prostatic adenocarcinoma as well its risk stratification on the basis of serum PSA, Gleason score and Grade grouping system.
Materials and Methods
After obtaining approval from Institutional Ethics Committee (IEC No-RKC/426), this hospital based observational cross-sectional study was carried out in the Department of Pathology, RG Kar Medical College, Kolkata. The core biopsies were taken from the lesions with the help of transrectal ultrasound and additional cores from suspected lesion as identified by digital rectal examination in the Department of Urology, RG Kar Medical College, Kolkata. All the core biopsies sent to the Department of Pathology from April 2017 to March 2018 were included in the study. However, those biopsy specimens where preoperative serum PSA level was unknown were excluded from the study. As per criteria, total 45 cases were included in the present study.
Prostatic core biopsies taken from different sites of prostate were sent in separate vials to the Department of Pathology for processing and histopathological reporting. To maintain the ideality for processing as well as reporting, 1-2 (maximum 3) different kinds of cores were embedded in a single cassette.
Cutting at several levels of biopsy core (at a maximum thickness of 4 μm) was done for each paraffin block as it increases the yield of a definite adenocarcinoma diagnosis. Each slide was stained by H&E stain. For positive cases of prostatic adenocarcinoma, the reporting format had been followed as per recommendation of The College of American Pathologist (CAP) [12] containing: 1) The Histological Type; 2) The number of cores positive for cancers and total no. of cores examined; 3) Linear cancer extent/ Highest percentage of cancer in a single core; 4) Gleason’s score as appreciated on H&E stained sections [2]; 5) Grade Grouping recommended by AJCC (American Joint Commission on Cancer), Eighth Edition. The current Grade Grouping is based on the histologic pattern of arrangement of carcinoma cells in haematoxylin and eosin stained sections. Five basic grade patterns are used to generate a histologic Gleason score that ranges from 1 to 5. Grade Group is the stratification of histologic grade scores into prognostically relevant groups: Grade Group 1 (Gleason score ≤6), Grade Group 2 (Gleason score 3+4=7), Grade Group 3 (Gleason score 4+3=7), Grade Group 4 (Gleason score 8), and Grade Group 5 (Gleason score 9-10) [13]. In the eighth edition of AJCC, it has been recommended that both the Gleason grade and the grade group should be used to determine tumor grade [2,14].
Statistical Analysis
The comparison of PSA value between benign and malignant cases along with its significance of association with higher Grade-Grouping was statistically judged by unpaired t-test using Graph Pad Quick Calcs software.
Results
In our study for one year, total numbers of TRUS guided prostatic core biopsy specimen were 45. Out of 45 cases 22 cases showed benign i.e., benign hyperplasia of prostate [Table/Fig-1] and 23 cases showed prostatic adenocarcinoma. So, the percentage of positivity of adenocarcinoma was 51.11%. Regarding the age group, the mean age group for benign cases were 63.77(±11.24) and for malignant cases 66.7(±4.9) showed in [Table/Fig-2].
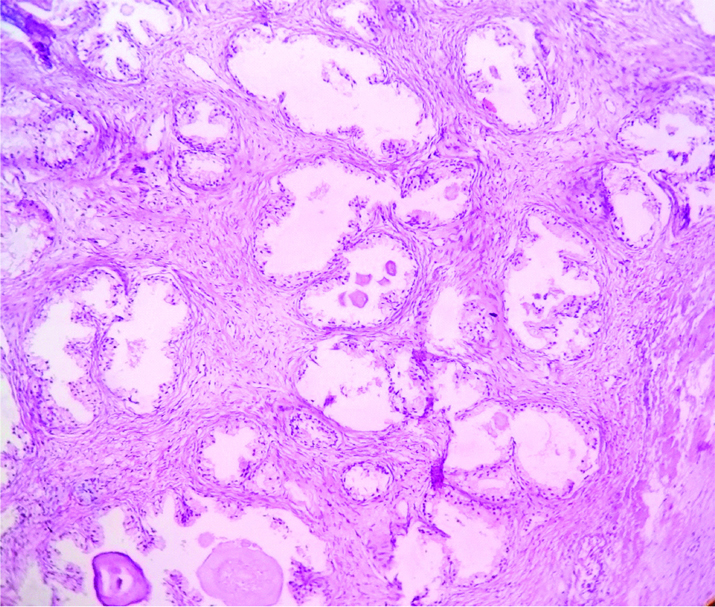
Photo micrograph showing picture of benign hyperplasia of prostate.(H&E 400X).
Comparison of age in benign and malignant group.
| Disease Group | Mean Age (±SD) | p-value |
|---|
| Benign (N=22) | 63.77(±11.24) | 0.2609 |
| AdenoCA (N=23) | 66.7(±4.9) |
This study showed mean PSA value (ng/mL) in benign cases as 3.45 (±0.987) and malignant cases it was 58.47 (±22.191) [Table/Fig-3] and the result was statistically significant (p-value<0.001).
Comparison of PSA value in benign and malignant group.
| Disease Group | Mean PSA (±SD) [ng/mL] | p-value |
|---|
| Benign (N=22) | 3.45 (±0.987) | <0.001 |
| AdenoCA (N=23) | 58.47 (±22.191) |
After analysis of prostatic adenocarcinoma cases [Table/Fig-4] on the basis of Gleason score and Group-Grading as shown in [Table/Fig-5], it was found that total 12 cases were in Gleason Score 7. Out of 12 cases, 4 cases (17.4%) showed histopathological Gleason score of (3+4) that belongs to Group Grading 2 and 8 cases (34.78%) showed histopathological Gleason score of (4+3), which belongs to Group Grading 3. This group which having Gleason score <8, actually belong to low to intermediate risk and mean PSA value 58.75 ng/mL. Regarding high risk group, 8 cases (34.78%) showed Gleason score 8(4+4) and 3 cases (13.04%) showed Gleason score 9 (5+4) with Group Grading 4 and 5 respectively. Mean PSA level was 58.18 ng/mL in this high risk group [Table/Fig-5,6].
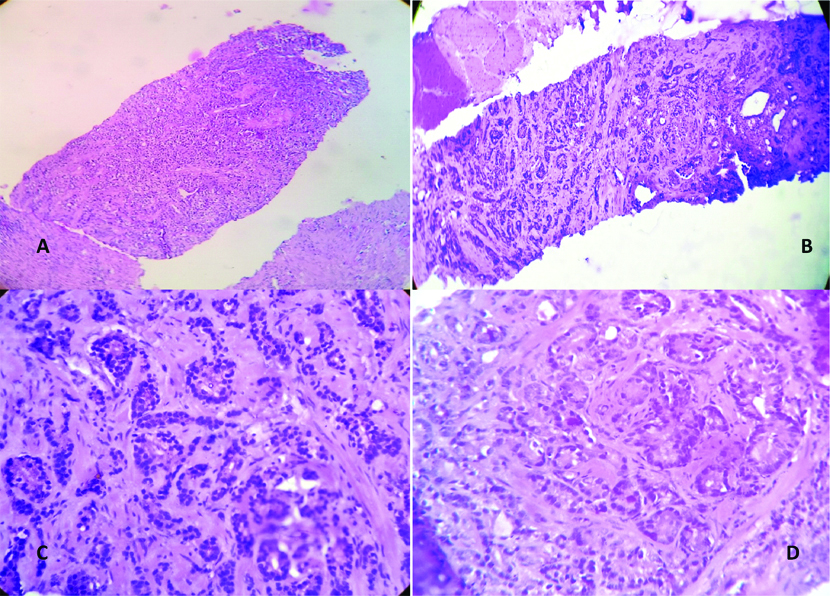
a) Photomicrograph showing prostatic adenocarcinoma in TRUS-guided biopsy, Gleason score (5+4)=9, Group Grade-5 (H&E 100X); b) Prostatic adenocarcinoma, Gleason Score (4+3)=7, Grade Group-3 (100X); c) Prostatic adenocarcinoma, GleasonScore (3+4)=7, Grade Group=2 (400X); d) Prostatic Adenocarcinoma, Gleason Score (4+4)=8, Grade Group (400X).
Distribution of malignant cases according to Gleason Score & AJCC Group Grading.
| Gleason Score | AJCC Group Grading | Number of Cases (%) |
|---|
| 7 (3+4) | 2 | 4 (17.4) |
| 7 (4+3) | 3 | 8 (34.78) |
| 8(4+4) | 4 | 8 (34.78) |
| 9 (5+4) | 5 | 3 (13.04) |
Comparison of PSA value between High risk (Gleason Score ≥8) and Low to Intermediate risk group (Gleason score <8).
| Risk Group | Mean PSA (±SD) (ng/mL) | p-value |
|---|
| Low to Intermediate (Gleason score <8) (N=12) | 58.75 (±26.43) | 0.95 |
| High (Gleason Score ≥8) (N=11) | 58.18 (±17.74) |
However, in malignant cases which have been categorised as low to intermediate risk group (Gleason score <8) and high risk group (Gleason score ≥8), the mean PSA values (ng/mL) are 58.75 and 58.18 respectively and these values were not statistically significant (p-value=0.95) to differentiate between these two groups.
Discussion
The prostatic carcinomas are typically a disease of men older than 50 years of age [14]. In our present study the mean age for adenocarcinoma of prostate was 66.7(±4.9). It is concordant with previously reported various studies done by Hariharan K et al., Huang TH et al., and Siegel R et al., [15-17]. Hariharan K et al., observed that peak age group for incidence of prostate cancer was above 65 years [15]. The study of Huang TH et al., revealed that patients younger than 50 years accounted for only 0.55% of all patients with prostate cancer [16]. In United States, Prostate carcinoma is the 3rd leading cause of death due to cancer in males in age group of 60 to 79 years [17]. Regarding Benign hyperplasia of prostate, it is also a disease of men of older age group with mean age group in different studies published by Lee YJ et al., and Yeboah Eet al., showed above 50 years [18,19]. In present study, the mean age for benign cases were 63.77 (±11.24) and statistically it is not helpful to differentiate with prostate cancer as p-value is 0.2609 [Table/Fig-2].
Serum PSA is a tumour marker but its serum levels are under the influence of physiological and pathological processes and hence PSA is not highly specific for prostate carcinoma. Clinically applicable reference values for this marker are from 0-4.0 ng/mL, but they don’t exclude carcinoma always. Intermediary PSA values, i.e., value between 4.0-10.0 ng/mL, can be present in patients with benign hyperplasia of prostate, prostatitis, intraepithelial neoplasia as well as in prostate carcinoma cases[20].
In present study, mean PSA value (ng/mL) in case of adenocarcinoma of prostate was 58.47(±22.191) while in case of benign lesions it was 3.45(±0.987). This is an interesting finding which shows that patients with markedly elevated serum PSA levels are more likely to harbour adenocarcinoma in their biopsies than benign changes, as in previous studies done by Dai B et al., [21]. So from this study it was proved that mean PSA has got definite significance in case of prostatic adenocarcinoma and the difference between mean PSA value in benign and malignant cases of prostate was statistically significant. However, Banerjee B et al., concluded in their article published in 2016 that chances of finding malignancy with increasing values of PSA are more, but not a rule. It can only give a clue to the histopathologist to examine the sections more thoroughly [22]. In contrast, Amayo A et al., concluded that although PSA is a sensitive test, it is not sufficiently specific to discriminate between Benign Prostate Hyperplasia (BPH) and Carcinoma of Prostate at intermediate values [23]. In fact, serum levels of PSA are elevated to a lesser extent in BPH than in prostatic carcinomas but there is considerable overlap [5]. It has been observed that in many cases of Benign hyperplasia of prostate the mean PSA are significantly high and in many cases of localized Prostatic adenocarcinoma the mean PSA are not significantly high. So nowadays not only the mean PSA but also PSA velocity and PSA density, age-specific reference range and percentage of free PSA are used as helpful and adjunctive parameters to differentiate between benign and malignant cases [5]. Higher level of PSA (>5.0 ng/mL) is associated with poorly differentiated histology (GS>7) and this is well documented [24]. Pierorazio P et al., showed in their retrospective study on 1932 patients that for an individual patient, the higher the initial PSA level the higher the risk of having poorly differentiated prostate cancer [24]. In our study most of the prostatic adenocarcinoma cases had Gleason score of intermediate to high grade, i.e., from 7 to 9. But the difference between the mean PSA levels was not statistically significant between low to intermediate grade group and high grade-group prostatic adenocarcinoma diagnosed by TRUS-guided prostatic biopsy.
Few previous studies also support our findings for example, Schroder FH et al., found that approximately half of the tumours missed with PSA 0 to 4 ng/mL had aggressive characteristics[25]. In 2015, GurumurthyD et al., conducted a study on 51 cases of prostate carcinoma where majority of the patients had poorly differentiated carcinoma and they found that though there was a comparative increase in PSA level with increase in Gleason grade, it was not statistically significant[26]. McGuire BB et al., also concluded in their study that in patients with Gleason grade 8-10, a proportion of tumors were so poorly differentiated that they produced relatively little PSA [27]. This might be explained by the fact that less differentiated tumours sometimes produce less PSA due to loss of phenotype expression of PSA, which follows dedifferentiation of tumour cells [20].
Serum PSA determination has certain limitations for the diagnosis of prostatic cancer. It is commonly elevated in benign prostatic hyperplasia and prostatitis, as well as with mechanical manipulation of the prostate gland [28]. These factors, coupled with the biological variation in PSA concentrations, result in low specificity and low positive predictive value when used as a single measurement [5].
However, serum PSA levels correlate strongly with the risk of prostate cancer [25,29]. The increase in serum PSA depends on differentiation of tumour cells. Gleason score and grade grouping are most powerful predictors of biological behaviour and influential factors used in determining treatment. PSA, when combined with Gleason score and clinical stage, improves the prediction of pathological stage for prostatic carcinoma [30].
Limitation
In the present setting, authors could not correlate the cases with other serum parameters like PSA velocity, PSA doubling time. Also any other immune-histochemical markers could not be used in addition to routine Haematoxylin-Eosin (H&E) stain for diagnosis of prostate adenocarcinoma. Further, Gleason scoring itself has certain limitations like Gleason Grade on needle biopsy specimen of prostate cancer may not match with final grade following radical prostatectomy.
Conclusion
This study shows that estimation of serum PSA has got definite significance in differentiating benign cases from malignant cases in prostatic core biopsy specimens (TRUS guided). But in cases of prostate adenocarcinoma while considering the disease stage based on Gleason Score and Grade-grouping, the serum PSA value has got little significance. However, further prospective studies with larger sample size may enlighten the controversies regarding this issue.
[1]. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Cancer Today (powered by GLOBOCAN 2018)IARC CancerBase No. 15. [Internet] 2018. [Cited November 28, 2018] Available from: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf [Google Scholar]
[2]. Humphrey PA, Amin MB, Berney DM, Billis A, Cao D, Cheng L, WHO Classification of Tumours of the Urinary, System and Male Genital Organs. 4th ed. LyonInternational Agency for Research on Cancer 2016 Acinar Adenocarcinoma. In: Moch H, Humphrey PA, Ulbright TM, Reuter VE, editors [Google Scholar]
[3]. Andreoiu M, Cheng L, (2010). Multifocal Prostate cancer: biologic, prognostic, and therapeuticimplicationsHum Pathol 2010 41(6):781-93.10.1016/j.humpath.2010.02.01120466122 [Google Scholar] [CrossRef] [PubMed]
[4]. Antenor JA, Han M, Roehl KA, Nadler RB, Catalana WJ, Relationship between initial prostate specific antigen level and subsequent prostate cancer detection in alongitudinal screening studyJ Urol 2004 172(1):90-93.10.1097/01.ju.0000132133.10470.bb15201744 [Google Scholar] [CrossRef] [PubMed]
[5]. Epstein JI, Loton TL, Robbins and Cotran Pathologic Basis of Disesae 2016 9th EdPhiladelphiaSaunders ElsevierThe Lower Urinary Tract and Male Genital System. In: Kumar V, Abbas AK, Fausto N, Aster JC, editors [Google Scholar]
[6]. Scher HI, Eastham JA, Harrisons Principles of Internal Medicine 2015 19th edNew YorkMcGraw-Hill educationBenign and Malignant Diseases of Prostate. In: Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL, Loscalzo J, editors [Google Scholar]
[7]. Esposti PL, Franzen S, Transrectal aspiration biopsy of the prostate. A re-evaluation of the method in the diagnosis of prostatic carcinomaScand J Urol Nephrol Suppl 1980 55:49-52. [Google Scholar]
[8]. Harvey CJ, Pilcher J, Richenberg J, Patel U, Frauscher F, Applications of transrectal ultrasound in prostate cancerBr J Radiol 2012 85 Spec No 1(Spec Iss 1):S3-17.10.1259/bjr/5635754922844031 [Google Scholar] [CrossRef] [PubMed]
[9]. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, Van der Kwast T, EAU guidelines on prostate cancer. Part 1. screening, diagnosis, and local treatment with curative intent-update 2013EurUrol 2014 65(1):124-37.10.1016/j.eururo.2013.09.04624207135 [Google Scholar] [CrossRef] [PubMed]
[10]. Hameed O, Humphrey PA, Immunohistochemistry in diagnostic surgical pathology of the prostateSemin Diagn Pathol 2005 22:88-104.10.1053/j.semdp.2005.11.00116512601 [Google Scholar] [CrossRef] [PubMed]
[11]. Toi A, Neill M, Lockwood G, Sweet J, Tammsalu L, Fleshner N, The continuing importance of transrectal ultrasound identification of prostatic lesionsJ Urol 2007 177:516-20.10.1016/j.juro.2006.09.06117222623 [Google Scholar] [CrossRef] [PubMed]
[12]. Paner G, Srigley J, Zhou M, Allan R, Amin M, Chang S, Cancer Protocol Templates | College of American Pathologists. [Internet]College of American Pathologists June 2017 [Cited 2018 Nov 24] Available from: https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates [Google Scholar]
[13]. Buyyounouski MK, Choyke PL, Kattan MW, McKenney JK, Srigley JR, Barocas DA, AJCC Cancer Staging Manual 2018 8th edChicagoAmerican College of SurgeonsProstate. In: Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al., editors [Google Scholar]
[14]. Buyyounouski MK, Choyke PL, McKenney JK, Sartor O, Sandler HM, Amin MB, Prostate cancer-major changes in the American Joint Committee on Cancer eighth edition cancer staging manualCA Cancer J Clin 2017 67:245-53.10.3322/caac.2139128222223 [Google Scholar] [CrossRef] [PubMed]
[15]. Hariharan K, Padmanabha V, Demography and disease characteristics of prostate cancer in IndiaIndian J Urol 2016 32:103-08.10.4103/0970-1591.17477427127351 [Google Scholar] [CrossRef] [PubMed]
[16]. Huang TH, Kuo JY, Huang YH, Chung HJ, Huang WJS, Wu HHH, Prostate cancer in young adults Seventeen-year clinical experience of a single centerJ Chin Med Assoc 2017 80:39-43.10.1016/j.jcma.2016.10.00427914715 [Google Scholar] [CrossRef] [PubMed]
[17]. Siegel R, Ma J, Zou Z, Jemal A, Cancer Statistics 2014CA Cancer J Clin 2014 64(1):9-29.10.3322/caac.2120824399786 [Google Scholar] [CrossRef] [PubMed]
[18]. Lee YJ, Lee JW, Park J, Seo S, Chung J, Yoo TK, Nationwide incidence and treatment pattern of benign prostatic hyperplasia in KoreaInvestig Clin Urol 2016 57(6):424-30.10.4111/icu.2016.57.6.42427847916 [Google Scholar] [CrossRef] [PubMed]
[19]. Yeboah E, Prevalence of benign prostatic hyperplasia and prostate cancer in Africans and Africans in the DiasporaJ West AfrColl Surg 2016 6(4):1-30. [Google Scholar]
[20]. Zivkovic S, Correlation between prostate-specific antigen and histopathological difference of prostate carcinomaArch Oncol 2004 12(3):148-51.10.2298/AOO0403148Z [Google Scholar] [CrossRef]
[21]. Dai B, Ye DW, Kong YY, Shen YJ, Wang BH, Individualized prostate biopsy strategy for Chinese patients with different prostate specific antigen levelsAsian J Androl 2008 10(2):325-31.10.1111/j.1745-7262.2008.00345.x18097514 [Google Scholar] [CrossRef] [PubMed]
[22]. Banerjee B, Iqbal BM, Kumar H, Kambale T, Bavikar R, Correlation between prostate specific antigen levels and various prostatic pathologiesJ Med Soc 2016 30:172-5.10.4103/0972-4958.191184 [Google Scholar] [CrossRef]
[23]. Amayo A, Obara W, Serum prostate specific antigen levels in men with benign prostatic hyperplasia and cancer of ProstateEast Afr Med J 2004 81(1):22-26.10.4314/eamj.v81i1.879015080511 [Google Scholar] [CrossRef] [PubMed]
[24]. Pierorazio P, Desai M, McCann T, Benson M, McKieman J, The relationship between preoperative prostate specific antigen and biopsy Gleason sum in men undergoing radical retropubic prostatectomy: a novel assessment of traditional predictors of outcomeBJU Int 2009 103(1):38-42.10.1111/j.1464-410X.2008.07952.x18778352 [Google Scholar] [CrossRef] [PubMed]
[25]. Schroder FH, van der Cruijsen-Koeter I, de Koning HJ, Vis AN, Hoedemaeker RF, Kranse R, Prostate cancer detection at low prostate specific antigenJ Urol 2000 163(3):806-12.10.1016/S0022-5347(05)67809-3 [Google Scholar] [CrossRef]
[26]. Gurumurthy D, Maggad R, Patel S, Prostate carcinoma: correlation of histopathology withserum prostate specific antigenSci J Clin Med 2015 4(4-1):1-5.10.11648/j.sjcm.s.2015040401.11 [Google Scholar] [CrossRef]
[27]. McGuire BB, Helfand BT, Loeb S, Hu Q, O’Brien D, Cooper P, Outcomes in patients with Gleason score 8-10 prostate cancer: relation to preoperative PSA levelBJU Int 2012 109(12):1764-69.10.1111/j.1464-410X.2011.10628.x22017732 [Google Scholar] [CrossRef] [PubMed]
[28]. Bratt O, Lilja H, Serum markers in prostate cancer detectionCurrOpin Ural 2015 25(1):59-64.10.1097/MOU.000000000000012825393274 [Google Scholar] [CrossRef] [PubMed]
[29]. Aus G, Damber JE, Khatami A, Lilja H, Stranne J, Hugosson J, Individualized screening interval for prostate cancer based on prostate-specific antigen level: results of a prospective, randomized, population-based studyArch Intern Med 2005 165(16):1857-61.10.1001/archinte.165.16.185716157829 [Google Scholar] [CrossRef] [PubMed]
[30]. Guimaraes MS, Quintal MM, Meirelles LR, Magna LA, Ferreira U, Billis A, Gleason score as predictor of clinicopathologic findings and biochemical (PSA) progression following radical prostatectomyInt. Braz J Urol 2008 34(1):23-29.10.1590/S1677-5538200800010000518341718 [Google Scholar] [CrossRef] [PubMed]