Insidious Role of Diabetes Mellitus on Nerves and Dental Pulp
Saramma Mathew Fenn1, Mohan Narayanan2, Mathew Jacob3
1 Senior Lecturer, Department of Oral Medicine and Radiology, Vinayaka Mission’s Sankarachariyar Dental College, Vinayaka Mission’s Research Foundation (Deemed to be University), Salem, Tamil Nadu, India.
2 Professor and Head, Department of Oral Medicine and Radiology, Vinayaka Mission’s Sankarachariyar Dental College, Vinayaka Mission’s Research Foundation (Deemed to be University), Salem, Tamil Nadu, India.
3 Reader, Department of Oral Pathology and Microbiology, Vinayaka Mission’s Sankarachariyar Dental College, Vinayaka Mission’s Research Foundation (Deemed to be University), Salem, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Saramma Mathew Fenn, Department of Oral Medicine and Radiology, Vinayaka Mission’s Sankarachariyar Dental College, Vinayaka Mission’s Research Foundation (Deemed to be University), Salem, Tamil Nadu, India.
E-mail: docere_saramathew@yahoo.co.in; drsarammamathewfenn@vmsdc.edu.in
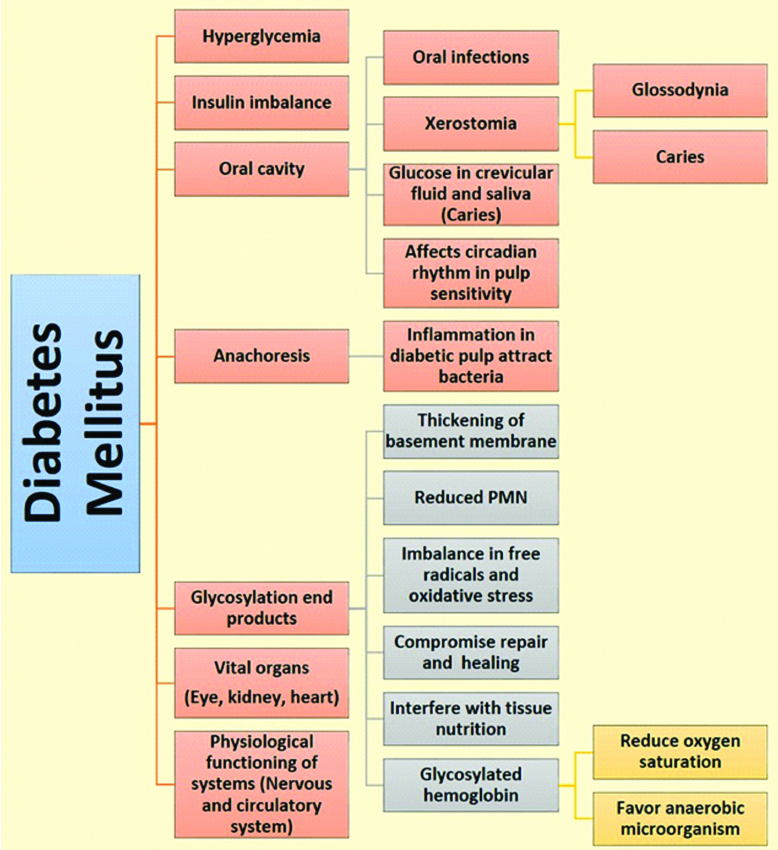
Diabetes Mellitus is a metabolic disorder with deleterious effect on the physiological functioning of body systems. The predisposition of body organs and tissues to infections, nervous and circulatory dysfunction brands this disease with a wide spectrum of clinical manifestations. The present scenario in the management of patients with diabetes has shifted from symptom-specific treatment to a holistic approach in the overall treatment of soft and hard tissue pathologies presented by the disease. A deeper comprehension of the pathogenesis of diabetic complications, both general and local, can serve as an ally in rendering a “tailored” treatment which not only alleviates the symptoms but treats the underlying cause. This article addresses the generalised effect of hyperglycaemia and its insidious but progressive effect on the dental pulp.
Anachoresis, Hyperglycaemia, Pulpal infections, Sensory nerve fibers
Introduction
Diabetes Mellitus is a clinically and genetically heterogeneous metabolic disease characterised by abnormally elevated blood glucose levels with dysregulation of carbohydrate, protein and lipid metabolism [1]. The gradual progression and insidious nature of the disease affect the physiological functioning of the body systems. The treatment of diabetes mellitus poses challenges in maintaining the normal level of blood glucose to delay the inevitable systemic complications. Among the body systems, the nervous and vascular systems are two of the earlier affected systems with severe debilitating consequences [2]. This article describes the effect of hyperglycaemia on the nervous system and the changes seen on the innervation of the dental pulp in diabetics.
Effect of Diabetes on Nerves
Loss of sensation in the extremities is one of the earliest complications to develop in diabetics. Diabetic patients have a high risk of developing neuropathy and elicit varying neurological symptoms such as numbness, dysesthesia, increased pain during the night, to loss of sensation with the progression of disease [3]. As a result, diabetics frequently present with painless ulcerations in lower limbs discovered as an incidental finding [4]. However, the relation of vascular abnormalities and nerve fibres deterioration is still being investigated. Development of abnormalities in vascular channels such as capillary basement membrane thickening, endothelial hyperplasia with diminished oxygen tension and hypoxia reduce the nerve conduction velocities resulting in decreased peripheral nerve sensation [5]. The link between vasculopathy and neuronal fibre degeneration are found to be proportional to the malfunctioning of the pancreatic gland and glycaemia. Regardless of the type of diabetes, it has been observed that all the aspects of the nervous system are eventually affected, with the sensory and autonomic system to be greatly affected than the motor system [6].
The duration and hyperglycaemic level correlate to the severity and damage to the nervous tissue. Diabetic neuropathy is mainly due to the alteration in the sorbitol or polyol pathway. The polyol pathway converts glucose to fructose utilising the enzymes aldose reductase and sorbitol dehydrogenase thereby reducing the accumulation of sorbitol within the cell. The failure of the enzymes results in deposition of sorbitol in the cell causing hypoxia leading to nervous tissue ischaemia [7]. Also, studies have shown the possibility that genetic alteration of the aldose reductase gene may also play a role in the nervous system damage in diabetics [8-11]. It has been shown that the longer nerves exhibit earlier loss of nerve sensation in diabetics. However, in the pulp, the long nerves demyelinate to form a plexus beneath the odontoblasts which extend their bodies into the dentin and by the hydrodynamic mechanism provide sensory perception. This unique sensory mechanism has been postulated to be affected in diabetes patients due to several factors such as increased inflammatory mediators, thickening of basement membrane and hampered collagen synthesis [12].
Oral Complications
The chronic elevation of blood glucose has shown to bring about long-term damage to vital organs including the oral cavity. The classical features of uncontrolled diabetes include a wide spectrum of oral manifestations such as xerostomia, opportunistic fungal infections and periodontitis. Several studies have shown a reduced salivary flow rate in patients with HBA1c greater than 7%, signifying an association of salivary gland function and low glycaemic control [13-15]. The effect of polyuria and hyperglycaemia on salivary gland parenchyma hampers the saliva production. The volume of fluid decreased in the extracellular component occurs due to polyuria, consequently reducing the volume of saliva and increasing the viscosity preventing the mechanical flushing action on retentive cariogenic bacteria residing on the surface of the tooth [16].
The normal salivary glucose (0.5-1.00 mg/100 mL) do not significantly affect oral health but higher values of salivary glucose favour the proliferation of microorganisms and enhance their colonisation on teeth and oral mucous membrane. Moreover, an increased level of glucose in saliva and crevicular fluid occurs due to an ineffective filtration of glucose from blood thus increasing the incidence of dental caries [17,18]. Altered taste sensation, one of the early signs of diabetic neuropathy, is an indicator of fluctuation in blood glucose levels and is a consequence of alteration in the taste bud receptors for glucose moieties [19].
Diabetes and Dental Pulp
Diabetes mellitus has a direct effect on the structural components of dental pulp. In a study conducted on diabetes-induced rats, histologically evident changes were observed in the structure and function of the blood vessels supplying the dental pulp. The morphometric analyses on these rats showed a significant reduction in volumetric density of collagen fibres and fibroblasts around blood vessels contributing to the progression of pulpal and periapical infections [20]. In uncontrolled and long-standing diabetics the dental pulp showed significant reduction in the blood flow of pulp due to thickening of the basement membrane of the blood vessel and reduced collateral circulation. With increased cell mediators such as kallikrein-nitrite levels in dental pulp, a diminished leukotactic response cause pulpal inflammation and irreversible damage (necrosis) to the dental pulp [21]. These changes in vasculature also reduce the oxygen supply and overall oxygen saturation favouring an environment for proliferation of anaerobic bacteria [22].
“Multifaceted” is one word that aptly describes the physiological functioning of the sensory nerve supplying the tooth. The uniqueness lies in the fact that innervation of the dental pulp not only provides innervation for sensation, but also protection and repair. The sensory innervation is performed by the polymodal nociceptors that respond to thermal, mechanical and chemical stimuli primarily by the myelinated A-delta and the partially unmyelinated C-fibres through the dentinal fluid dynamics. It has been observed that the nerves of the tooth undergo structural and morphological changes during inflammation in the dental pulp (visualised under an electron microscope) with fibres that slip between the odontoblastic bodies, twist around the tubules sprouting nerve fibres which express strong positivity of S100 markers for macrophages or dendritic cell [23].
Numerous studies have established a significant correlation between high blood glucose levels and duration of diabetes on the nervous system [24]. The peripheral nerve cells in diabetics’ undergo glycosylation exerting direct toxicity on the nerves along with endoneurial microangiopathy. Increase in glucose entry within a cell cause release of oxidants in mitochondria leading to reduced mitochondrial action potential with poor energy synthesis decreasing the overall conduction potential of nerves. These are clinically translated as decrease in response to any stimuli by electrical stimulation in dental pulp or stimuli to vibration or touch in the peripheral parts of the body [25].
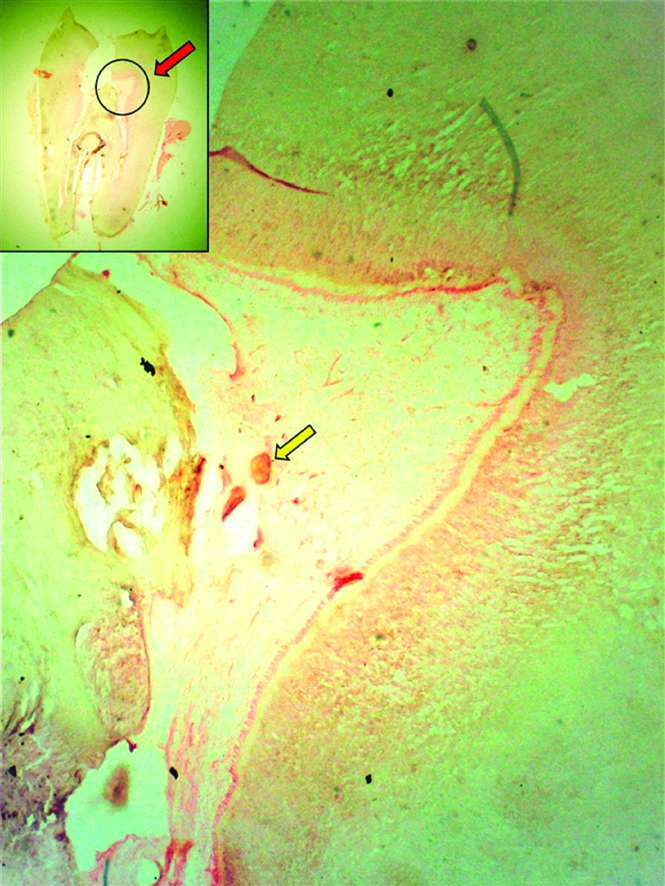
Pulp stones are age-related physiological calcifications frequently identified in pulp chambers of elderly. Pulps of diabetics are more prone to such calcifications and have been linked to elevated hyperglycaemia, duration of diabetes and late diabetic vascular changes in uncontrolled diabetics [26,27] [Table/Fig-1]. Another distinct feature is the “Sickle” shaped appearance of the pulp stones when compared to globular or spherical laminations or stratifications observed in non-diabetics [28].
Longitudinal Ground Section of permanent molar (Inset) showing a Decalcified section of tooth with Pulp stone (Yellow arrow).
Predisposition to Caries, Pulpal Infections and Periodontitis
Pulps of unrecognised diabetes and uncontrolled diabetes are always in a state of perpetual inflammation due to impairment in sensory nerve activity and microcirculation causing necrosis of pulp. “Diabetic odontalgia” is a condition frequently encountered in diabetic patients with longstanding hyperglycaemia presenting with symptoms of pain similar to pulpitis but in absence of caries. The condition usually affects a single tooth however cases with multiple teeth involvement in two or more quadrants have also been reported. Such patients may also exhibit a negative response in electric pulp testing for vitality. These conditions are often resolved when patients are treated for the underlying unrecognised diabetes and usually the pulpal infections subside when insulin therapy is initiated with a favourable response to endodontic treatment [29].
Apart from the crown, the other means by which cariogenic bacteria access the pulp is by the process known as Anachoresis. By this route, microorganisms present in the bloodstream from other sources along with the toxins end up in the pulp inducing pulpal and periapical inflammation in diabetics [Table/Fig-2]. These bring about asymptomatic pulpal and periapical infections described by Bender IB et al., [28]. Numerous case reports have also observed in unrecognised cases of diabetes a higher prevalence of periapical pathosis, osteolytic lesions and delay in periapical repair without any clinical or radiographic evidence of caries [30-32].
Effect of Diabetes mellitus on the body tissues, vital organs and oral cavity.
There are many factors identified for loss of teeth in diabetics and advanced glycation products in gingiva and periodontium have been identified as the primary reason of weakening of the attachment of root to the socket wall. The other reason is the reduced function of neutrophils promoting periodontal disease when compared to healthy individuals [33]. Diabetes is a risk factor for severe periodontal disease as the periodontopathic organisms amplify the magnitude of the advanced glycation end product-mediated cytokine response in diabetes mellitus [34].
Conclusion
In a nutshell, the “silent killer” disease is a challenging scenario for dentists as patients with diabetes often report in advanced stages of caries or periodontal diseases due to delayed response. The mechanism of this systemic disease, the effect of diabetes on the innervation of the tooth and surrounding structures has to be understood by the dentist for effective management and treatment.
[1]. Ship JA, Diabetes and oral health: an overviewJ Am Dent Assoc 2003 134 Spec No:4S-10S.10.14219/jada.archive.2003.036718196667 [Google Scholar] [CrossRef] [PubMed]
[2]. Forbes JM, Cooper ME, Mechanisms of diabetic complicationsPhysiol Rev 2013 93(1):137-88.10.1152/physrev.00045.201123303908 [Google Scholar] [CrossRef] [PubMed]
[3]. Mohan G, Chandey M, Monga A, Dev P, Comparative study of detection of diabetic neuropathy by clinical and nerve conduction study in type 2 diabetes mellitus patientsInt J Adv Med 2018 5:380-83.10.18203/2349-3933.ijam20181073 [Google Scholar] [CrossRef]
[4]. Syafril S, Pathophysiology diabetic foot ulcerIOP Conf. Ser.: Earth Environ. Sci 2018 125(1):1-6.10.1088/1755-1315/125/1/012161 [Google Scholar] [CrossRef]
[5]. Shamikh JB, Oral manifestations in controlled and uncontrolled diabetic patients-a study in JordanPak Oral Dent J 2012 2(3):456-59. [Google Scholar]
[6]. Lamontagne A, Buchthal F, Electrophysiological study in diabetic neuropathyJ Neurol Neurosurg Psychiatry 1970 33(4):442-52.10.1136/jnnp.33.4.4425505671 [Google Scholar] [CrossRef] [PubMed]
[7]. Hosseini A, Abdollahi M, Diabetic neuropathy and oxidative stress: therapeutic perspectivesOxid Med Cell Longev 2013 2013:16803910.1155/2013/16803923738033 [Google Scholar] [CrossRef] [PubMed]
[8]. Heesom AE, Millward A, Demaine AG, Susceptibility to diabetic neuropathy in patients with insulin dependent diabetes mellitus is associated with a polymorphism at the 5’ end of the aldose reductase geneJ Neurol Neurosurg Psychiatry 1998 64:213-16.10.1136/jnnp.64.2.2139489533 [Google Scholar] [CrossRef] [PubMed]
[9]. Sivenius K, Pihlajamaki J, Aldose reductase gene polymorphisms and peripheral nerve function in patients with type2 diabetesDiabetes Care 2004 27(8):2021-26.10.2337/diacare.27.8.202115277434 [Google Scholar] [CrossRef] [PubMed]
[10]. Tang WH, Martin K, Aldose reductase, oxidative stress and diabetic mellitusFront Pharmacol 2012 3:8710.3389/fphar.2012.00087 [Google Scholar] [CrossRef]
[11]. Gupta B, Singh SK, Association of aldose reductase gene polymorphism(C-106T) in susceptibility of diabetic peripheral neuropathy among north Indian populationJ Diabetes Complications 2017 31(7):1085-89.10.1016/j.jdiacomp.2017.04.01128495421 [Google Scholar] [CrossRef] [PubMed]
[12]. Fowler MJ, Microvascular and macrovascular complications of diabetesClinical Diabetes 2008 26(2):77-82.10.2337/diaclin.26.2.77 [Google Scholar] [CrossRef]
[13]. Lasisi TJ, Fasanmade AA, Salivary flow and composition in diabetic and non-diabetic subjectsNiger J Physiol Sci 2012 27(1):79-82. [Google Scholar]
[14]. Prathiba KM, Johnson P, Ganesh M, Subhashini AS, Evaluation of Salivary Profile among Adult Type 2 Diabetes Mellitus Patients in South IndiaJ Clin Diagn Res 2013 7(8):1592-95.10.7860/JCDR/2013/5749.323224086848 [Google Scholar] [CrossRef] [PubMed]
[15]. Morais EF, Macedo RA, Lira JA, de Lima KC, Borges BC, Factors related to dry mouth and low salivary flow rate in diabetic elderly: A systematic literature reviewRev Bras Geriatr Gerontol 2014 17(2):417-23.10.1590/S1809-98232014000200018 [Google Scholar] [CrossRef]
[16]. Panda A, Venkatapathy R, Nirima O, Glucose estimation in the salivary secretion of diabetes mellitus patientsDiabetes, Metab Syndr Obes 2012 5:149-54.10.2147/DMSO.S3211222923999 [Google Scholar] [CrossRef] [PubMed]
[17]. Leite MF, Ganzerla E, Marques MM, Nicolau J, Diabetes induced metabolic alterations in dental pulpJ Endod 2008 34(10):1211-14.10.1016/j.joen.2008.07.01018793922 [Google Scholar] [CrossRef] [PubMed]
[18]. Panchbhai AS, Correlation of salivary glucose level with blood glucose level in diabetes mellitusJ Oral Maxillofac Res 2012 3(3):e310.5037/jomr.2012.330324422015 [Google Scholar] [CrossRef] [PubMed]
[19]. Bhandare NN, Keny MS, Ramnath P, Diabetic tongue-could it be a diagnostic criterion?J Family Med Prim Care 2014 3(3):290-91.10.4103/2249-4863.14165425374875 [Google Scholar] [CrossRef] [PubMed]
[20]. Claudino M, Nunes IS, Gennaro G, Cestari TM, Spadella CT, Garlet GP, Diabetes triggers the loss of tooth structure associated to radiographical and histological dental changes and its evolution to progressive pulp and periapical lesions in ratsArch Oral Biol 2015 60(11):1690-98.10.1016/j.archoralbio.2015.08.01526355529 [Google Scholar] [CrossRef] [PubMed]
[21]. Catanzaro O, Dziubecki D, Lauria LC, Ceron CM, Rodriguez R, Diabetes and its effects on pulpJ Oral Sci 2006 48(4):195-99.10.2334/josnusd.48.19517220616 [Google Scholar] [CrossRef] [PubMed]
[22]. Lima SM, Grisi DC, Kogawa EM, Franco OL, Peixoto VC, Goncalves-Junior JF, Diabetes mellitus and inflammatory pulpal and periapical disease: a reviewInt Endod J 2013 46(8):700-09.10.1111/iej.1207223442003 [Google Scholar] [CrossRef] [PubMed]
[23]. Manolea H, Vasile N, Opri M, Fronie A, Popescu MR, Immunohistochemical and electronmicroscopy aspects of the nerve structures from the dental pulpRom J Morphol Embryol 2014 55(1):147-52. [Google Scholar]
[24]. Kakrani AL, Gokhale VS, Vohra KV, Chaudhary N, Clinical and Nerve Conduction Study Correlation in Patients of Diabetic NeuropathyJ Assoc Physicians India 2014 62(1):24-27. [Google Scholar]
[25]. Yagihashi S, Mizukami H, Sugimoto K, Mechanism of diabetic neuropathy: Where are we now and where to go?J Diabetes Investig 2011 2(1):18-32.10.1111/j.2040-1124.2010.00070.x24843457 [Google Scholar] [CrossRef] [PubMed]
[26]. Vibhute NA, Vibhute AH, Daule RT, Bansal PP, Mahalle A, Hard facts about stones: pulpal calcifications: a reviewJ Pat Care 2016 2:10510.4172/2573-4598.1000105 [Google Scholar] [CrossRef]
[27]. Inagaki Y, Yoshida K, Ohba H, Seto H, Kido J, Haneji T, Nagata T, High glucose levels increase osteopontin production and pathologic calcification in rat dental pulp tissuesJ Endod 2010 36(6):1014-20.10.1016/j.joen.2010.03.01820478457 [Google Scholar] [CrossRef] [PubMed]
[28]. Bender IB, Bender AB, Diabetes mellitus and the dental pulpJ Endod 2003 9(6):383-89.10.1097/00004770-200306000-0000112814220 [Google Scholar] [CrossRef] [PubMed]
[29]. Kim S, Neurovascular interactions in the dental pulp in health and inflammationJ Endod 1990 16(2):48-53.10.1016/S0099-2399(06)81563-3 [Google Scholar] [CrossRef]
[30]. Marotta PS, Fontes TV, Armada L, Lima KC, Rocas IN, Siqueira JF Jr, Type 2 diabetes mellitus and the prevalence of apical Periodontitis and endodontic treatment in an adult Brazilian populationJ Endod 2012 38(3):297-300.10.1016/j.joen.2011.11.00122341063 [Google Scholar] [CrossRef] [PubMed]
[31]. Segura-Egea JJ, Castellanos-Cosano L, Machuca G, Lopez-Lopez J, Martin-Gonzalez J, Velasco-Ortega E, Sanchez-Domniguez B, Diabetes mellitus, periapical inflammation and endodontic treatment outcomeMed Oral Patol Oral Cir Bucal 2012 17(2):e356-61.10.4317/medoral.1745222143698 [Google Scholar] [CrossRef] [PubMed]
[32]. Lopez-Lopez J, Jane-Salas E, Estrugo-Devesa A, Velasco-Ortega E, Martin-Gonzalez J, Segura-Egea JJ, Periapical and endodontic status of type 2 diabetic patients in Catalonia, Spain: a cross-sectional studyJ Endod 2011 37(5):598-601.10.1016/j.joen.2011.01.00221496655 [Google Scholar] [CrossRef] [PubMed]
[33]. Albrecht M, Banoczy J, Tamas G Jr, Dental and oral symptoms of diabetes mellitusCommunity Dent Oral Epidemiol 1988 16(6):378-80.10.1111/j.1600-0528.1988.tb00586.x3203498 [Google Scholar] [CrossRef] [PubMed]
[34]. Grossi SG, Genco RJ, Periodontal disease and diabetes mellitus: a two-way relationshipAnn Periodontol 1998 3(1):51-61.10.1902/annals.1998.3.1.519722690 [Google Scholar] [CrossRef] [PubMed]