Case Series
Case 1
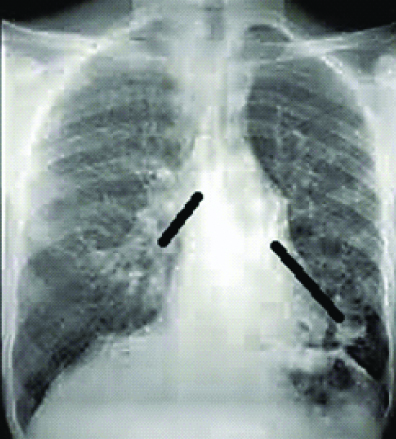
An 18 year-old boy presented with productive cough, fever and haemoptysis off and on, for over a month. He denied weight loss, chest pain or breathlessness. There was no history of Pulmonary Tuberculosis (PTB), diabetes or other immunocompromised conditions. He revealed casual contact with cats. On examination, he was febrile, tachypneic, oxygen saturation of 96% on room air by pulse oximetry and haemodynamically stable. Auscultation revealed coarse crepitations over the right mammary area. White Blood Cell (WBC) count was 9.3x103/μL with neutrophilia. Blood glucose, renal function, electrolytes and liver function were normal. Chest radiograph showed ill-defined infiltrates and cystic opacities in the right mid lung zone [Table/Fig-1]. Sputum sample was sent for Ziehl-Neelsen stain for Acid Fast bacilli (AFB), gram stain and bacterial culture.
Chest radiograph showing ill-defined infiltrates and cystic opacities in the right mid lung zones.

Empiric therapy with intravenous ceftriaxone (3 g/day) was started. But, the initial clinical response was poor and he continued to be febrile. Sputum was negative for AFB and gram staining revealed gram negative coccobacilli. Bacterial cultures were negative. Computed Tomography (CT) thorax showed right middle lobe cystic bronchiectasis. In view of negative cultures and poor clinical response, a bronchoscopy was performed and Bronchoalveolar Lavage (BAL) was subjected for AFB and bacterial culture. BAL was inoculated on blood agar, chocolate agar and MacConkey’s agar, followed by incubation at 37°C for 18-24 hours. Dew drop, non-haemolytic colonies appeared on blood agar and small dew drop colonies on chocolate agar. MacConkey’s agar showed no growth. The isolate was catalase and oxidase positive and subsequently identified as P. canis by the VITEK-2 system (bioMérieux, Marcy-l’Etoile, France). Antibiotic susceptibility testing by modified Kirby Bauer method showed susceptibility to azithromycin and ciprofloxacin. Antibiotic therapy was switched to oral ciprofloxacin (1 g/day). After 72 hours of treatment, the patient was afebrile, haemoptysis subsided and WBC count was 7.1x103/μL. Ciprofloxacin was continued for two weeks and follow-up chest radiographs suggested resolution.
Case 2
A 60 year-old female was admitted for productive cough, fever and increased breathlessness, of five days duration. There was no haemoptysis or chest pain. She was a known case of Chronic Obstructive Pulmonary Disease (COPD) and bronchiectasis, with recurrent exacerbations of COPD. She underwent treatment for PTB six years back. She did not have diabetes or other immunocompromised conditions. There was no animal contact. On physical examination, she was febrile, tachypneic, oxygen saturation of 86% on room air with extensive crepitations in the lung bases. Chest radiograph showed hyperinflated lungs with bilateral lower lung zone cystic opacities [Table/Fig-2]. Blood tests revealed a WBC count of 17x103/μL with neutrophilia. Blood glucose, renal and liver functions were normal.
Chest radiograph showing hyper inflated lung fields with bilateral lower zone cystic opacities.
She was treated with injection amoxicillin/clavulanic acid (3.6 g/day), oxygen and bronchodilators. But, the initial clinical response was poor and WBC count increased to 16.8×103/μL. Sputum was negative for AFB and gram staining revealed gram negative coccobacilli. Sputum was inoculated on blood agar, chocolate agar and MacConkey’s agar, followed by incubation at 37°C for 18-24 hours. Dew drop, non-haemolytic colonies appeared on blood agar and small dew drop colonies on chocolate agar. MacConkey’s agar showed no growth. The isolate was catalase and oxidase positive and subsequently identified as P. canis by the VITEK-2 system. Antibiotic susceptibility testing by modified Kirby Bauer method showed susceptibility to ciprofloxacin, levofloxacin and trimethoprim/sulfamethoxazole. Antibiotics were switched to oral ciprofloxacin (1 g/day) and trimethoprim/sulfomethoxazole (320/1600 mg/day). After 72 hours of treatment, the patient was afebrile, and WBC count was 13.1×103/μL. She was discharged after five days in good health and antibiotics were continued for two weeks. Follow-up visits suggested stable respiratory symptoms.
Case 3
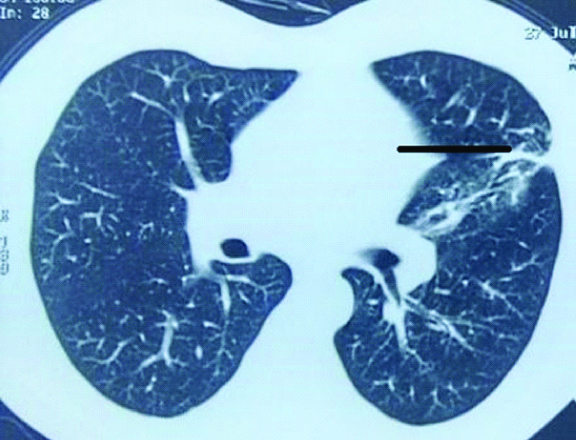
A 55 year-old male presented with productive cough and fever of two weeks’ duration. He denied haemoptysis, breathlessness or chest pain. He was a known case of chronic lymphocytic leukemia diagnosed three years back. The disease relapsed three months back and fludarabine and cyclophosphamide based chemotherapy was started. There was no history of PTB, diabetes or animal contact. On physical examination he was febrile, tachypneic and crepitations over left hemithorax. Chest radiograph suggested ill-defined opacities in the left mid-lung zone. Blood tests revealed neutropenia (WBC count: 3×103/μL) and thrombocytopenia (platelets: 54×103/μL). Blood glucose, renal and liver functions were normal. A thoracic CT revealed fibrosis with traction bronchiectasis in left upper lobe [Table/Fig-3].
HRCT showing traction bronchiectasis in left upper lobe.
He was treated empirically with injection cefaperazone/sulbactam (4g/day) and amikacin (0.75 g/day). Sputum was negative for AFB and gram staining revealed gram negative coccobacilli. Sputum was inoculated on blood agar, chocolate agar and MacConkey’s agar, followed by incubation at 37°C for 18-24 hours. Dew drop, non-haemolytic colonies appeared on blood agar and small dew drop colonies on chocolate agar. MacConkey’s agar showed no growth. The isolate was catalase and oxidase positive and subsequently identified as P. canis by the VITEK-2 system. Antibiotic susceptibility testing by modified Kirby Bauer method showed susceptibility to azithromycin. He was treated with oral azithromycin (500 mg/day). The patient responded symptomatically and azithromycin was continued for two weeks. Follow-up chest radiographs suggested resolution.
Case 4
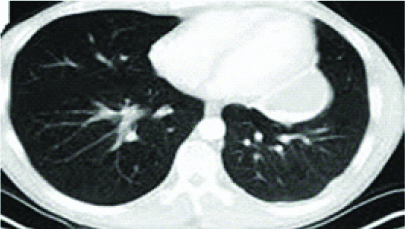
A 61 year-old female presented with non-productive cough, breathlessness and fever of one month duration. She was a known case of Rheumatoid arthritis, on oral methotrexate and hydroxychloroquine for last six months. There was no history of PTB, diabetes or other immunocompromised conditions. She denied animal contact. On physical examination, she was afebrile, haemodynamically stable and crepitations over the right mammary area. Chest radiograph was normal. Blood tests revealed a WBC countof 9×103/μL with neutrophilia. Blood sugar, renal and liver parameters were normal. Although chest radiograph was normal, a CT thorax was performed in view of possible lung involvement in rheumatoid arthritis. Thoracic CT revealed centrilobular nodules with tree-in-bud appearance in right upper and middle lobe and left upper lobe [Table/Fig-4a].
HRCT showing centrilobular nodules with tree-in-bud appearance in right upper and middle lobe and left upper lobe.

Empirical antibiotic therapy with injection ceftazidime (4 g/day) was started. Sputum induction with 3% saline was attempted with little success. In view of scanty expectoration and radiological features suspicious of PTB, bronchoscopy was performed and BAL was subjected for AFB and bacterial culture. The sample was negative for AFB by smear and Xpert MTB/RIF. Gram staining revealed gram negative coccobacilli. BAL was inoculated on blood agar, chocolate agar and MacConkey’s agar, followed by incubation at 37°C for 18-24 hours. Dew drop, non-haemolytic colonies appeared on blood agar and small dew drop colonies on chocolate agar. MacConkey’s agar showed no growth. The isolate was catalase and oxidase positive and subsequently identified as P. canis by the VITEK-2 system. Antibiotic susceptibility testing by modified Kirby Bauer method showed susceptibility to amoxicillin/clavulanic acid and ciprofloxacin. Antibiotics were switched to oral amoxicillin/clavulanic acid (2 g/day) and ciprofloxacin (1 g/day). She responded symptomatically and antibiotics were continued for two weeks. Follow HRCT after four weeks showed clearance of centrilobular nodules [Table/Fig-4b]. Follow-up visits suggested a stable clinical condition.
HRCT showing clearance of centrilobular nodules.
Case 5
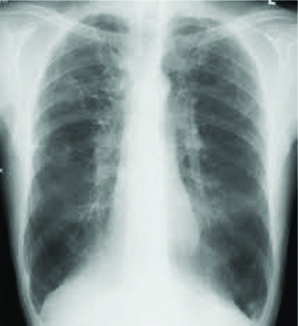
A 65 year-old male presented with productive cough, fever and increased breathlessness of two weeks duration. He was a known case of COPD and diabetes mellitus on treatment with inhaled bronchodilators and oral hypoglycaemic drugs. He had multiple COPD exacerbations and glycaemic control was poor. There was no history of PTB or other immunocompromised conditions. He denied animal contact. On physical examination, he was febrile, tachypneic, oxygen saturation of 88% on room air and had bilateral expiratory wheeze. A chest radiograph showed hyperinflated lung fields [Table/Fig-5]. Blood tests revealed a WBC count of 8.5×103/μL with neutrophilia, a fasting blood glucose level of 195 mg/dL and glycol haemoglobin of 8.5%. Renal and liver parameters were normal.
Chest radiograph showing hyper inflated lung fields.
He was treated with injection ceftriaxone (3g/day), oxygen, inhaled bronchodilators and insulin. Sputum was sent for AFB stain and bacterial culture. Initial response to treatment was poor, had haemoptysis and WBC count increased to 15.7×103/μL. Bacterial culture and AFB smear was negative. Thoracic CT showed left lower lobe cystic bronchiectasis. Bronchoscopy was performed in view of haemoptysis and BAL was sent for AFB and bacterial culture. The sample was negative for AFB by smear and Xpert MTB/RIF. Gram staining revealed gram negative coccobacilli. BAL was inoculated on blood agar, chocolate agar and MacConkey’s agar, followed by incubation at 37°C for 18-24 hours. Dew drop, non-haemolytic colonies appeared on blood agar and small dew drop colonies on chocolate agar. MacConkey’s agar showed no growth. The isolate was catalase and oxidase positive and subsequently identified as P. canis by the VITEK-2 system. Antibiotic susceptibility testing by modified Kirby Bauer method showed susceptibility topenicillin, amoxicillin/clavulanic acid, ciprofloxacin, levofloxacin and trimethoprim/sulfomethoxazole. Antibiotic therapy was switched to injection amoxicillin/clavulanic acid (3.6 g/day) and levofloxacin (500 mg/day). After 72 hours of treatment, the patient was afebrile, haemoptysis subsided and TLC was 8.3×103/μL. He was discharged on oral amoxicillin/clavulanic acid and levofloxacin for two weeks along with insulin and inhaled bronchodilators. Subsequent follow-up suggested a stable clinical course.
Discussion
Pasteurella species are known to cause a variety of human infections. Skin, eye and soft tissue are the most common sites of pasturella infections in humans [1,2]. Few cases of meningitis, endocarditis, intra-abdominal infections and septicaemia have been reported [3]. Systemic involvement is exceptional, evidenced by few case reports. Respiratory involvement can manifest as sinusitis, tracheobronchitis, pneumonia, lung abscess or empyema [4]. Pneumonia is commonly seen in patients with underlying lung disease like chronic sinusitis, COPD, or bronchiectasis [5,6]. A case has been described in a patient with Pasturella canis infection leading to left sided pneumonia with para pneumonic pleural effusion [7]. It is usually lobar with a short pro-drome. But cases of multilobar and diffuse pulmonary infections have been described [8]. Empyema caused by P. multocida was described by Nelson SC et al., [8]. Life threatening systemic infection can occur, especially in immunocompromised hosts [9].
An interesting observation from our study was in the majority of our patient no obvious history of contact with pets could be traced and all had an underlying chronic lung disease like bronchiectasis and COPD with systemic cause for immune-compromised status like diabetes mellitus, hematologic malignancy and immunosuppressive therapy. The culture isolates were mostly from bronchial lavage specimens. This probably implies that P. canis infections could be more common than previously believed, but are reported less due to diagnostic challenges. In a tuberculosis endemic country like India, these zoonotic pneumonias can be misdiagnosed for tuberculosis and treated empirically with anti-tubercular medications. Literature on P. canis infections other than skin and soft tissue involvement is sparse. Pneumonia caused by P. canis in COPD patients has been reported [10,11]. Dual infection with P. dagmatis and P. canis in a 25-year-old female with dog bite wound infection was described [12]. P. canis was reported as a cause for peritonitis in a patient undergoing peritoneal dialysis [13]. Conjunctivitis caused by P. canis was reported [14]. P. canis has been associated with bacteremia in a cirrhotic patient with open leg wound caused by an animal bite [15].
Penicillin has emerged as the drug of choice in all forms of Pasteurella infections [16]. Third generation cephalosporins like ceftriaxone and cefaperazone have also shown in-vitro activity against Pasteurella species. Beta-lactamase producing strains in humans have been reported, mostly in respiratory isolates and thought to have acquired resistance from beta-lactamase producing strains of Haemophilus influenza present concomitantly in the respiratory tract [17,18]. Penicillin resistance may be associated with an ROB-1 beta-lactamase encoding plasmid, very similar to that found in some Haemophilus species [19]. Among the non-beta lactam antibiotics, tetracyclines, fluoroquinolones, macrolides like azithromycin, trimethoprim/sulfomethoxazole and chloramphenicol have demonstrated good in-vitro activity. Drugs like aminoglycosides, clindamycin and erythromycin have moderate to poor activity and are not recommended.
Most of our cases were susceptible to ciprofloxacin and one case was exclusively susceptible to azithromycin. Although penicillin susceptibility is the rule, resistance was observed in two cases. Further analysis of isolates for beta lactamase production was not performed. Ciprofloxacin was used in combinations with amoxicillin/clavulanic acid and trimethoprim/sulfamethoxazole with good clinical response. Although the duration of treatment is not well defined, a 14-day course was given to all patients, guided by previous reports. Ten to fourteen days of therapy appears a reasonable duration and tailored to clinical improvement. Although most cases respond well, persons with underlying lung disease can develop significant complications with reported mortality rates between 20-30% [20]. All our patients improved well with none developing septicaemia.
Conclusion
Pasteurella canis is a rare cause of pneumonia, and reported mainly in persons with underlying lung disease and immunocompromised conditions. Clinicians and microbiologists should have awareness and high index of suspicion of such exceptional infections in patients at risk. A detailed patient history about animal exposure is of paramount importance for the diagnosis of such infections. There also is a strong indication for diagnostic bronchial lavage and cultures in such cases of refractory pneumonia. Avoidance of animal contact is a simple measure for preventing such infections. As these organisms are frequently susceptible to routine antibiotics, early diagnosis and appropriate treatment may prevent untoward complications.
Abbreviations
AFB: Acid Fast Bacilli; BAL: Bronchoalveolar Lavage; CLL: Chronic Lymphocytic Leukaemia; COPD: Chronic Obstructive Pulmonary Disease; CT: Computed Tomography; PTB: Pulmonary Tuberculosis; WBC: White Blood Cell.
Declaration
Ethics approval and consent to participate: The study proposal was approved by the institutional ethics committee.
Consent for publication: Written informed consent was obtained from the patients for the publication of this case series.
Availability of data and materials: The data sets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests: The authors declare that they have no competing interests.
Authors’ contribution: All the authors contributed equally to: 1) the interpretation of the clinical data, 2) the drafting of the manuscript and revising it critically, and 3) the final approval of the submitted manuscript.
[1]. Bongyoung K, Hyunjoo P, Kwang-hyun L, Yangsoon L, Identification of Pasteurella canis in a soft tissue infection caused by a dog bite: The first report in KoreaAnn Lab Med 2016 36:617-19.10.3343/alm.2016.36.6.61727578520 [Google Scholar] [CrossRef] [PubMed]
[2]. Sanjay S, Rupa M, Neeta G, Pasteurella canis biovar 2 causing dacryocystitis in HIV patient Journal of Clinical and Diagnostic Research 2017 11(2):DD01-DD03. [Google Scholar]
[3]. Weber DJ, Wolfson JS, Swartz MN, Hooper DC, Pasteurella multocida infections. Report of 34 cases and review of literatureMedicine 1984 63(3):133-54.10.1097/00005792-198405000-000016371440 [Google Scholar] [CrossRef] [PubMed]
[4]. Klein NC, Cunha BA, Pasteurella multocida pneumoniaSeminRespir Infect 1997 12(1):54-56. [Google Scholar]
[5]. Bartley EO, Pasteurella septica in chronic nasal sinusitisLancet 1960 2(7150):581-82.10.1016/S0140-6736(60)91647-0 [Google Scholar] [CrossRef]
[6]. Cawson RA, Talbot JM, The occurrence Pasteurella septica (syn. multocida) in bronchiectasisJ ClinPathol 1955 8(1):49-51.10.1136/jcp.8.1.4914354029 [Google Scholar] [CrossRef] [PubMed]
[7]. Ana Fa, Sara P, Pedro S, Filipa C, Susana F, Human infection by Pasteurella canis- A case reportBiomed J 2017 2(2):63-65.10.1016/j.pbj.2017.01.005 [Google Scholar] [CrossRef]
[8]. Nelson SC, Hammer GS, Pasteurella multocida empyema: Case report and review of the literatureAm J Med Sci 1981 281(1):43-49.10.1097/00000441-198101000-000077468640 [Google Scholar] [CrossRef] [PubMed]
[9]. Stein AA, Fialk MA, Blevins A, Armstrong D, Pasteurella multocida septicaemia. Experience at a cancer hospitalJAMA 1983 249(4):508-09.10.1001/jama.1983.033302800540306848852 [Google Scholar] [CrossRef] [PubMed]
[10]. Allison K, Clarridge JE 3rd, Long-term respiratory tract infection with canine-associated Pasteurella dagmatis and Neisseria canis in a patient with chronic bronchiectasisJ Clin Microbiol 2005 43(8):4272-74.10.1128/JCM.43.8.4272-4274.200516081998 [Google Scholar] [CrossRef] [PubMed]
[11]. Bhat S, Acharya PR, Biranthabail D, Rangnekar A, Shiragavi S, A case of lower respiratory tract infection with canine-associated Pasteurella canis in a patient with chronic obstructive pulmonary diseaseJ ClinDiagn Res 2015 9(8):DD03-04.10.7860/JCDR/2015/13900.635126435948 [Google Scholar] [CrossRef] [PubMed]
[12]. Akahane T, Nagata M, Matsumoto T, Murayama N, Isaka A, Kameda T, A case of wound dual infection with Pasteurella dagmatis and Pasteurella canis resulting from a dog bite-limitation of Vitek-2 system in exact identification of Pasteurella speciesEur J Med Res 2011 16(12):531-36.10.1186/2047-783X-16-12-53122112359 [Google Scholar] [CrossRef] [PubMed]
[13]. Castellano I, Marín JP, Gallego S, Mora M, Rangel G, Suarez MA, Pasteurella canis peritonitis in a peritoneal dialysis patientPerit Dial Int 2011 31(4):503-04.10.3747/pdi.2011.0000721799062 [Google Scholar] [CrossRef] [PubMed]
[14]. Balikogl-Yilmaz M, Yilmaz T, Esen AB, Engin KN, Taskapili M, Pasteurella canis and Granulicatella adiacens conjunctivitis outbreak resistant to empirical treatment in a child welfare agencyJ PediatrOphthalmol Strabismus 2012 49(5):314-19.10.3928/01913913-20120710-0222800794 [Google Scholar] [CrossRef] [PubMed]
[15]. Albert TJ, Stevens DL, The first case of Pasteurella canis bacteremia: A cirrhotic patient with an open leg woundInfection 2010 38(6):483-85.10.1007/s15010-010-0040-120623245 [Google Scholar] [CrossRef] [PubMed]
[16]. Stevens DL, Higbee JW, Oberhofer TR, Everett ED, Antibiotic susceptibilities of human isolates of Pasteurella multocidaAntimicrob Agents Chemother 1979 16(3):322-24.10.1128/AAC.16.3.322507787 [Google Scholar] [CrossRef] [PubMed]
[17]. Naas T, Benaoudia F, Lebrun L, Nordmann P, Molecular identification of TEM-1 beta-lactamase in a Pasteurella multocida isolate of human originEur J ClinMicrobiol Infect Dis 2001 20(3):210-13.10.1007/PL00011254 [Google Scholar] [CrossRef]
[18]. Lion C, Lozniewski A, Rosner V, Weber M, Lung abscess due to beta-lactamase-producing Pasteurella multocidaClin Infect Dis 1999 29(5):1345-46.10.1086/31343910525000 [Google Scholar] [CrossRef] [PubMed]
[19]. Livrelli V, Peduzzi J, Joly B, Sequence and molecular characterization of the ROB-1 beta-lactamase gene from Pasteurella haemolyticaAntimicrob Agents Chemother 1991 35(2):242-51.10.1128/AAC.35.2.2422024956 [Google Scholar] [CrossRef] [PubMed]
[20]. Breen D, Schonell M, Au T, Reiss-Levy E, Pasteurella multocida: A case report of bacteremic pneumonia and 10-year laboratory reviewPathology 2000 32(2):152-53.10.1080/00313020010444810840839 [Google Scholar] [CrossRef] [PubMed]