Introduction
Visfatin is a 52 kDa multifaceted molecule, which was detected and recognised as a new adipocytokine by Fukuhara A et al., in 2005 [1]. The name “visfatin” is used to refer to this protein as it is present in visceral fat and produced by adipose tissue. It is also known as Pre-B-cell Colony-Enhancing Factor (PBEF) or Nicotinamide Phosphoribosyl Transferase (NAMPT). Visfatin is originally produced by visceral fat tissue in obese animals and humans [1,2]. It is associated with obesity and with Type 2 Diabetes Mellitus (T2DM) [3] and has an important role in pancreatic β-cell function [4] and in the production of inflammatory cytokines [5]. NAMPT is the rate-limiting enzyme in the retrieve pathway of Nicotinamide Adenine Dinucleotide (NAD) biosynthesis from nicotinamide. As visfatin is regulating the NAD metabolism so, it might control fundamental cellular processes [6]. It also activates insulin receptors and has insulin-mimetic effects [7]. Obesity is widely heterogeneous and includes >30% of the obese population are metabolically healthy [8]. The pathogenesis of MS in adults is complex. Therefore, the link between circulating level of visfatin and MS is difficult to establish. However, several studies reporting that MS patients have higher concentrations of visfatin than normal subjects [9,10]. Insulin resistance leads to MS, which is activated by inflammatory cytokines such as IL-6 [11]. Adipose tissue behaving as an endocrine organ produce adipocytokines [12]. As visfatin is secreted by visceral fats, it has been assumed that its levels are associated with the amount of visceral fats. However, no consistent results have been obtained yet [13,14].
Several different studies reported the relationship between obesity and increased serum levels of oxidative stress markers. More cell damage in obese cases was associated with oxidative stress due to the increased oxygen utilisation and consequent radical formation through mitochondrial respiration, [15]. Some authors reported that the antioxidant enzymes activity was increased in obesity [16], while others found lower antioxidant enzymes activity in obese subjects compared to control [17]. Vitamin E is a lipid-soluble vitamin with lipid antioxidant properties [18]. Serum levels of α-tocopherol <11.6 μmol/L is defined as vitamin E deficiency [19]. It has been studied that vitamin E deficiency is associated with reduced insulin secretion and antioxidant capacity [20]. Hence, there is a possibility that vitamin E might affect the glucose metabolism independent of its antioxidant mechanism [21]. Likewise, vitamin A is involved in numerous metabolic processes in addition to its well-known role as an antioxidant [22]. Malondialdehyde (MDA) is formed during oxidation process of Polyunsaturated Fatty Acids (PUFA) by ROS [24]. Moreover, visfatin might promote premature endothelial cell death and stimulate DNA damage.
The purpose of this study was to assess the relationship between serum visfatin levels, oxidative stress markers, inflammatory marker (IL-6) and MS components in obese premenopausal women. In addition, assess the potential of the serum visfatin as a predictive marker of the MS in obese women and investigate its role in DNA damage and pregnancy complications.
Materials and Methods
Study Population
Sample size: This study is a cross-sectional study. Sample size was calculated based on the estimated prevalence of the disorder, population size and the confidence level was 1.96, which corresponds to a 95% confidence interval.
Inclusion criteria: The study included 25-35 years old, 150 premenopausal obese women and 80 non-obese healthy controls (regardless of the marital status). The obese patients were further divided into two groups: 75 with MS and 75 without MS. Obese women were patients at the obesity clinic at the National Research Centre (NRC), Egypt. MS was defined as having three or more criteria according to the modified NCEP ATP III definition [25].
Exclusion criteria: Authors excluded the women with history of cardiovascular disease or diabetes, hypothyroidism, pregnancy or under any medication known to affect glucose levels, insulin secretion, or insulin sensitivity and smoking.
Ethical Approval
The research was approved by the Ethical Committee of NRC (No: 16361) and followed the World Medical Association’s Declaration of Helsinki. Furthermore, each participant in the study signed a written consent after a full description of the study.
Anthropometric Measurements
Anthropometric parameters including calculated Body Mass Index (BMI) as weight in kilograms divided by height in meters square (kg/ m2) and Waist Circumference (WC) have been performed. Blood pressure was precisely measured as well.
Biochemical Measurements
The blood samples were collected after overnight fasting and stored at 80°C until further analysis. Enzymatic colourimetric analysis was carried out using Hitachi auto-analyser 704 (Roche Diagnostics Switzerland). Fasting plasma glucose, serum insulin concentration and serum lipids have been measured as per protocol previously described by Zaki M et al. [26].
Insulin resistance was estimated using Homeostatic Model Insulin Resistance (HOMA-IR) [27]. Enzyme linked immunosorbent assay (ELISA) kits provided by Glory Science were used to measure serum visfatin and IL-6 levels. The level of MDA, as an indicator of the lipid peroxidation, was determined by measuring Thiobarbituric Acid Reactive Species (TBARS) [28].
The TAC was measured in plasma spectrophotometrically. Serum TAC levels were determined using an automated measurement method that is based on the bleaching of the distinctive colour of 2,2- azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) (Beckman Coulter-Fullerton, CA, USA) [29]. The results were recorded in mmol Trolox equivalents/L. Vitamins A and E analyses were carried out using high performance liquid chromatography (HPLC) [29].
Determination of DNA Damage by Comet Assay
The comet assay was performed on blood samples of MS cases and healthy controls. The assay was performed following the previous method described in Blasiak J et al., [30]. As per Zakiet M et al., protocol, the assay included three major steps of analysis include peripheral blood leucocytes preparation followed by preparation of cell microgels on slides and finally visualisation and the analysis of the comet slides [31]. The present authors aimed of this study to assess the DNA damage in obese women with MS. Methods; 30 obese women with MS aged 25\u201335 years and thirty age-matched healthy non-obese women were enrolled in the study. Leukocyte DNA damage was assessed by comet assay. Results Among the obese women with MS, 20% of cases met criteria for Polycystic Ovary Syndrome (PCOS). The slides were examined at 400X magnification using a fluorescence microscope equipped with an excitation filter of 549 nm and a barrier filter of 590 nm. Evaluation of the degree of damage was done visually by scoring of the comet images. Duplicate slides were prepared for each case and one hundred cells were examined per case.
Statistical Analysis
The statistical analysis was done using the Statistical Package for the Social Sciences (SPSS) Version 16.0 for Windows (SPSS Inc). The normality was calculated by Kolmogorov-Smirnov test and the results were represented as Mean±SD. The distribution data of serum visfatin was skewed and logarithmically transformed values were used for all analyses. Mann-Whitney U-test was used in the analysis of DNA damage. In order to test the differences in baseline characteristics between the three groups (MS+, MS-, controls) one-way analysis of variance (ANOVA) has been used then, post-hoc tests were applied.
Multiple regression analysis by using stepwise linear regression with log visfatin as the dependent variable and metabolic, oxidative and inflammation parameters as the independent variables analysis was performed to assess the relationship between them in premenopausal obese women. The efficacy of the visfatin in distinguishing groups of cases with MS versus cases without was assessed by using Receiver Operating Characteristic (ROC) analysis. p-values <0.05 were considered to be statistically significant for all analyses.
Results
Significant differences (p<0.05) in biochemical and anthropometric parameters and visfatin levels between the three groups (with MS, without MS, controls) were observed. Results revealed that MS patients had significantly higher levels of visfatin, Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Fasting Blood Glucose (FBG), HOMA-IR, TC, TG, Low-Density Lipoprotein-Cholesterol (LDL-L), MDA and lower levels of TAC, vitamins A and E compared to the control group [Table/Fig-1]. Obese MS patients also showed significantly higher levels of visfatin, MDA and significant lower TAC than those without MS. Obese cases without MS showed significantly higher BMI and WC than controls.
Comparison of clinical and biochemical characteristics of the study groups.
| Characteristics | Obese With MS Without MS | Control | p-valuea |
|---|
| Age (years) | 31.8±4.5 | 31.3±3.5 | 32.3±4.3 | 0.67 |
| BMI (kg/m2) | 33.24±5.5* | 28.24±4.2$ | 21.24±3.2 | <0.05 |
| WC (cm) | 96.2±8.15* | 91.7±5.2$ | 80.7±4.2 | <0.01 |
| SBP (mmHg) | 135.8±10.7* | 132.3±8.4 | 100.3±6.2 | <0.01 |
| DBP (mmHg) | 85.8±7.9* | 70.21±7.6 | 71.24±8.8 | <0.01 |
| HOMA-IR | 6.7±1.2* | 3.8±1.4 | 2.8±1.3 | <0.01 |
| FBG (mg/dL) | 103.4±14.7 | 99.0±10.9 | 97.0±8.9 | <0.01 |
| TC (mg/dL) | 259.3±16.2* | 214.6±10.01 | 199.6±9.8 | <0.01 |
| TG (mg/dL) | 225.8±23.34* | 215.2±19.32 | 115.2±16.34 | <0.01 |
| HDL-C (mg/dL) | 65.6±4.9* | 48.84±11.7 | 43.84±10.1 | <0.01 |
| LDL-C (mg/dL) | 163.89±25.88* | 153±20.88 | 119.1±18.34 | <0.01 |
| Log visfatin (ng/mL) | 1.56±0.69*# | 1.40±0.51 | 0.92±0.41 | <0.01 |
| TAC (mmol/L) | 1.62±0.25*# | 1.82±0.23 | 1.92±0.28 | <0.01 |
| MDA (μmol/L) | 7.65±1.33*# | 4.39±0.12 | 3.15±0.25 | <0.01 |
| Serum vitamin A (μmol/L) | 0.81±0.03* | 0.91±0.13 | 2.29±0.93 | <0.01 |
| Alpha-tocopherol (μmol/L) | 13.3±3.12* | 14.3±3.99 | 15.3±4.12 | <0.01 |
| IL-6 (pg/mL) | 2.95±3.96* | 2.63±1.23 | 1.63±0.99 | <0.01 |
aFor statistical analysis, one-way ANOVA test between cases with MS, cases without MS and control, *p<0.05 Indicates significant differences vs. control, #p<0.05 Indicates significant differences vs. without MS.
BMI: Body mass index; WC: Waist circumference; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; TG: Triglyceride; FBG: Fasting blood glucose; HOMA-IR: Homeostasis model assessment of insulin resistance; TAC: Total antioxidant capacity. Values are presented as Mean±SD
The results of multiple stepwise regressions show positive co-relation of visfatin level with MS components, IL-6 and MDA, DNA damage, serum lipids, various measures of obesity and negative correlation with TAC and vitamins A and E [Table/Fig-2]. Comet assay was used to analyse DNA damage in combined cases of MS and pre-eclampsia. The damaged cells appeared as a fluorescent comet with tail comprising of DNA with strand breaks [Table/Fig-3].
Multiple stepwise regression analysis with log visfatin as a dependent variable in obese women.
| Variable | R | p-value |
|---|
| HOMA-IR | 0.44 | 0.01*** |
| FBG (mg/dL) | 0.35 | 0.02** |
| TC (mg/dL) | 0.31 | 0.01*** |
| TG (mg/dL) | 0.36 | 0.01*** |
| HDL-C (mg/dL) | -0.37 | 0.04** |
| LD-C (mg/dL) | 0.33 | 0.05* |
| SBP (mmHg) | 0.47 | 0.03** |
| DBP (mmHg) | 0.36 | 0.04** |
| WC (cm) | 0.49 | 0.01*** |
| MDA (μmol/L) | 0.41 | 0.04** |
| TAC | -0.41 | 0.03** |
| Vitamin A (μmol/L) | -0.45 | 0.02** |
| Alpha-tocopherol (mmol/L) | -0.34 | 0.03** |
| IL-6 (pg/mL) | 0.32 | 0.02** |
| DNA damage | 0.33 | 0.02** |
Multiple linear regression analysis, p<0.001,**p<0.01, *p<0.05
HOMA-IR: Homeostasis model assessment of insulin resistance; FBG: Fasting blood glucose; TC: Total cholesterol; TG: Triglyceride; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; WC: Waist circumference; MDA: Malondialdehyde; TAC: Total antioxidant capacity; ***IL-6: Interleukin-6
DNA damage in a case with combined MS and pre-eclampsia, evaluated by the comet assay. The damaged cell is visualised as cells with the appearance of a comet (arrow) showing a brightly fluorescent head and a tail to one side formed by the DNA containing strand breaks.
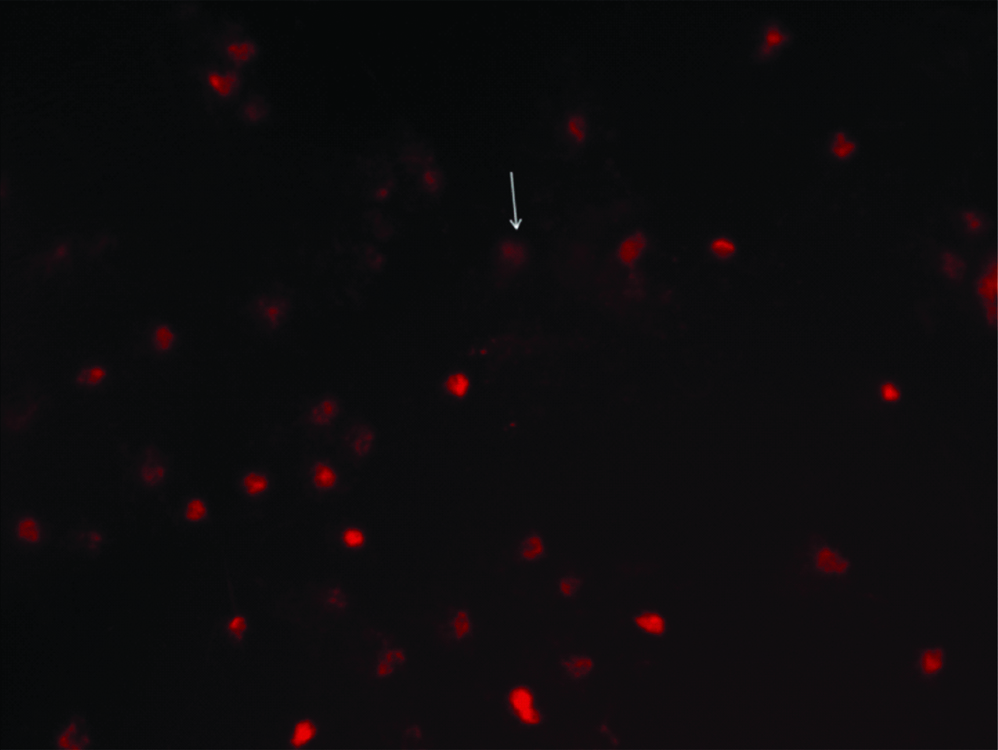
The mean percent of damage was significantly higher in obese MS women with pre-eclampsia history of as well as in MS without pre-eclampsia compared to controls (p<0.002). The mean frequency of DNA damage was 33.55±3.69 in MS women with history of pre-eclampsia, 25.95±2.16 in MS women without history of pre-eclampsia and 12.81±1.94 in controls with higher levels in MS cases with pregnancy complications than those without [Table/Fig-4]. This might indicate that MS causes DNA damage that in turn leads to other problems and complications.
Mean DNA damage in MS patients and controls.
| Group | Damage frequency Mean±SE | Mann-Whitney U-test |
|---|
| MS with recurrent pre-eclampsia | 33.55±3.69** | 0.002 |
| MS without recurrent pre-eclampsia | 25.95±2.16* |
| Control | 12.81±1.94 |
MS: Metabolic syndrome
*p<0.05, **p<0.01 vs. controls
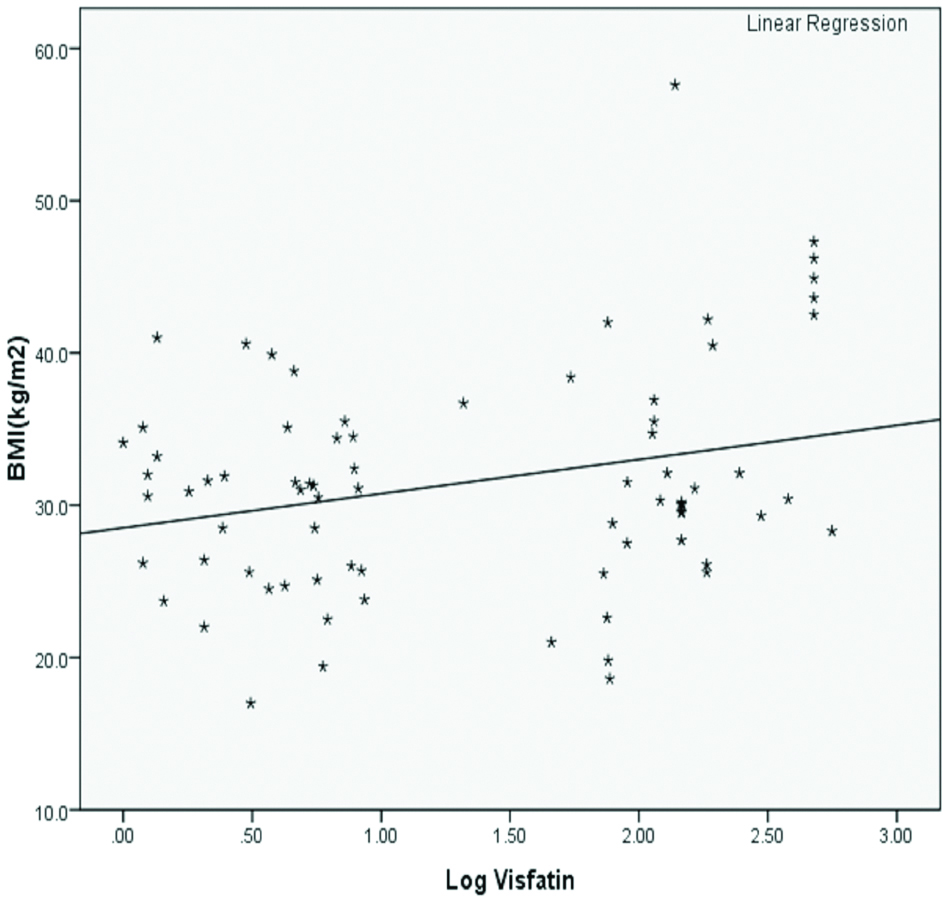
Positive correlation between log visfatin and BMI (kg/m2) in obese women with MS, indicates rise in visfatin levels along with the increase of BMI [Table/Fig-5].
Positive correlation between log visfatin and BMI (kg/m2) in obese women with MS.
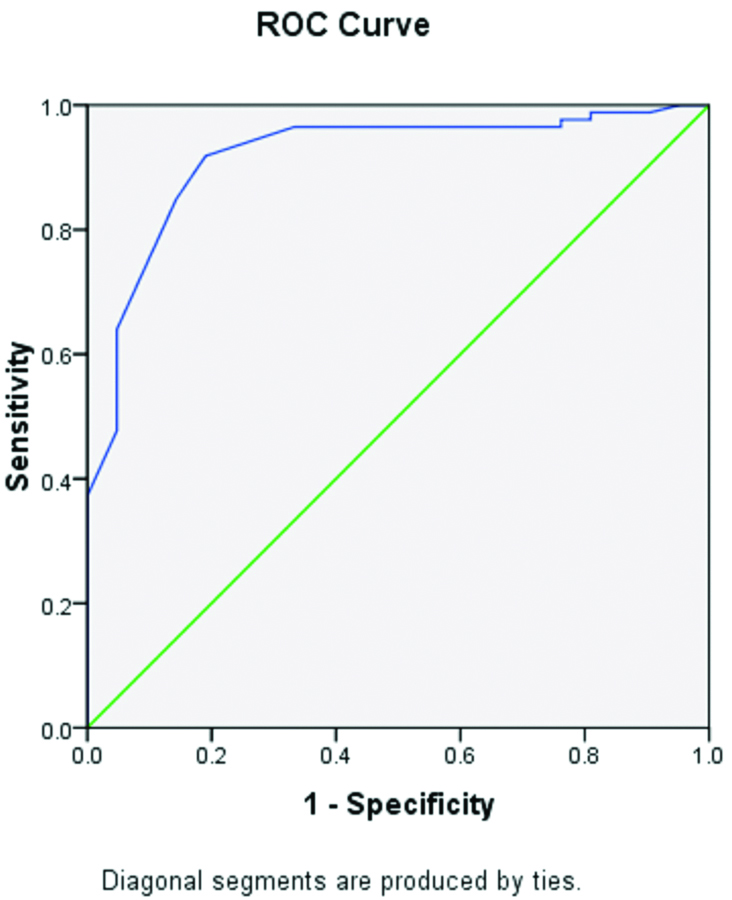
The cut-off of the log visfatin was 1.56 with 76% sensitivity and 71% specificity for detecting MS in obese women and the Area Under Curve (AUC) was 0.91 (p<0.001) [Table/Fig-6]. According to the results of the ROC analysis, visfatin level could be a good predictor of the MS in obese subjects.
Receiver operating characteristic curve of the log visfatin in detecting the presence of MS in obese women.
Area under ROC curve=0.91, p=0.001
Discussion
This study is among the first to determine the optimal visfatin threshold for diagnosing the MS in Egyptian obese women. The findings demonstrate the optimal visfatin cut-off point for diagnosis of MS in obese women. The previous study on Egyptian obese children and adolescents reported that cut-off point of visfatin for obesity was 5.5 and for MS in obese with a sensitivity of 86.1% and a specificity of 86.5% and an AUC of 0.93 [32]. In the present study, the cut-off of the log visfatin was 1.56 with 76% sensitivity and 71% specificity for detecting MS in obese women and the AUC was 0.91 (p<0.001). In this study, Authors also observed that women with pre-eclampsia had elevated oxidative stress and DNA damage; these findings were similar to the results obtained by Hilali N et al., [33]. Additionally, in the present study higher visfatin levels was detected in women with pre-eclampsia which is in agreement with Fasshauer M et al., 2007 and 2008 study where elevated visfatin levels have been reported in pre-eclampsia and other pregnancy complications [34,35].
Visfatin molecule has multifaceted features whose circulating levels are enhanced in metabolic disorders [5]. In the present study, significantly higher levels of visfatin have been found in obese MS cases relative to both control and obese cases without MS. The present results also indicated that serum concentration of visfatin increases along with the increasing BMI and are correlated positively with WC and lipid parameters. The results of the present study are in agreement with previous studies done by Kim JA et al., and Filippatos TA et al., that showed higher levels of visfatin in MS cases as compared to controls [36,37]. However, there have been contradictory results in the association between visfatin and obesity. Some of the studies found that serum visfatin levels are associated with obesity and visceral fat and not with subcutaneous fat when certificating sex and age-matched subjects [38]. Moreover, high visfatin levels in obese patients were found to be reduced after weight loss [12]. Conversely, Shoelson SE et al., found that visfatin levels were significantly lower in obese subjects [11]. On the other hand, Berndtey J et al., in another study found a positive correlation between visfatin levels and measures of obesity as WC and BMI [2].
Oxidative Stress is associated with unbalanced levels of adipokines involved in the development of the MS [39]. It happens as a result of the high production of pro-oxidants that exceeds the ability of the antioxidant system to remove them from circulation [40]. Central adiposity may contribute to OS due to visceral fat deposition and higher free-fatty acid flux through the portal circulation. In addition, peripheral insulin resistance may be associated with mitochondrial dysfunction and overproduction of pro-oxidants that can harm or alter proteins and lipids [40-41]. It has been suggested that oxidative stress is increased due to fat accumulation in MS patients [42]. Dyslipidemia and insulin resistance, which are associated with MS, increase the production of reactive oxygen species and consequently raise the oxidation of lipid products, MDA and proteins, which lead to endothelium dysfunction, cancer and other chronic diseases [43]. The present results indicated an increase in serum concentrations of visfatin in obese MS patients, associating with rising of MDA and lowering of TAC as compared to obese without MS and controls. There are some studies looking at the changes in TAC and MDA levels during certain metabolic conditions such as MS and obesity [44,45]. Association between oxidative stress and insulin resistance has been previously reported by Evans JL [46]. The increase of the plasma visfatin level in obese women has been previously reported by Zahorska-Markiewicz B et al., he observed significant higher visfatin levels in obese women compared to normal weight women which is similar with the present findings [47]. In the present study, authors also found positive association between visfatin serum concentrations and DNA damage as previously observed by Villalobos LA et al., who reported that visfatin promotes DNA damage [48]. It has been proposed that insulin secretion is regulated by visfatin levels and acts as an immune-modulator cytokine and involves in the inflammatory responses [49]. Elevated visfatin levels have been noticed in diabetic patients which might indicate faulty visfatin signalling or as a result of hyperglycemia or hyperinsulinemia [50]. Chronic low-grade inflammation that often accompanies the MS is a major factor in the mechanism of the MS and its consequent complications [51]. Oxidative stress markers, visfatin and IL-6 levels might yield new facts of pathways of the MS and the medical consequences of obesity such as acute coronary syndromes and atherosclerosis [52].
Limitation
This was a single-centred cross-sectional study, a larger multi-centric study should be done in future.
Conclusion
The present study found an association between visfatin levels, MS and oxidative stress. Authors suggest that serum visfatin could be used as a predictive marker for MS in obese subjects and its higher levels are associated with oxidative stress, DNA damage, IL-6 and pregnancy complications.
aFor statistical analysis, one-way ANOVA test between cases with MS, cases without MS and control, *p<0.05 Indicates significant differences vs. control, #p<0.05 Indicates significant differences vs. without MS.
BMI: Body mass index; WC: Waist circumference; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; TG: Triglyceride; FBG: Fasting blood glucose; HOMA-IR: Homeostasis model assessment of insulin resistance; TAC: Total antioxidant capacity. Values are presented as Mean±SD
Multiple linear regression analysis, p<0.001,**p<0.01, *p<0.05
HOMA-IR: Homeostasis model assessment of insulin resistance; FBG: Fasting blood glucose; TC: Total cholesterol; TG: Triglyceride; HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; WC: Waist circumference; MDA: Malondialdehyde; TAC: Total antioxidant capacity; ***IL-6: Interleukin-6
MS: Metabolic syndrome
*p<0.05, **p<0.01 vs. controls
[1]. Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Visfatin: a protein secreted by visceral fat that mimics the effects of insulinScience 2005 307(5708):426-30.10.1126/science.109724315604363 [Google Scholar] [CrossRef] [PubMed]
[2]. Berndt J, Klöting N, Kralisch S, Kovacs P, Fasshauer M, Schön MR, Plasma visfatin concentrations and fat depot-specific mRNA expression in humansDiabetes 2005 54(10):2911-6.10.2337/diabetes.54.10.291116186392 [Google Scholar] [CrossRef] [PubMed]
[3]. García-Fuentes E, García-Almeida JM, García-Arnés J, García-Serrano S, Rivas-Marín J, Gallego-Perales JL, Plasma visfatin concentrations in severely obese subjects are increased after intestinal bypassObesity 2007 15(10):2391-5.10.1038/oby.2007.28417925464 [Google Scholar] [CrossRef] [PubMed]
[4]. Rabe K, Lehrke M, Parhofer KG, Broedl UC, Adipokines and insulin resistanceMol Med 2008 14(11-12):74110.2119/2008-00058.Rabe19009016 [Google Scholar] [CrossRef] [PubMed]
[5]. Romacho T, Sánchez-Ferrer CF, Peiró C, Visfatin/Nampt: an adipokine with cardiovascular impactMediators Inflamm 2013 2013:94642710.1155/2013/94642723843684 [Google Scholar] [CrossRef] [PubMed]
[6]. Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesisEur J Immunol 2002 32(11):3225-34.10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L [Google Scholar] [CrossRef]
[7]. Galassi A, Reynolds K, He J, Metabolic syndrome and risk of cardiovascular disease: a meta-analysisAm J Med 2006 119(10):812-9.10.1016/j.amjmed.2006.02.03117000207 [Google Scholar] [CrossRef] [PubMed]
[8]. Blüher M, The distinction of metabolically “healthy” from “unhealthy” obese individualsCurr Opin Lipidol 2010 21(1):38-43.10.1097/MOL.0b013e3283346ccc19915462 [Google Scholar] [CrossRef] [PubMed]
[9]. Chen C-C, Li T-C, Li C-I, Liu C-S, Lin W-Y, Wu M-T, The relationship between visfatin levels and anthropometric and metabolic parameters: association with cholesterol levels in womenMetabolism 2007 56(9):1216-20.10.1016/j.metabol.2007.04.01817697864 [Google Scholar] [CrossRef] [PubMed]
[10]. Zhong M, Tan H, Gong H, Wang S, Zhang Y, Zhang W, Increased serum visfatin in patients with metabolic syndrome and carotid atherosclerosisClin Endocrinol (Oxf) 2008 69(6):878-84.10.1111/j.1365-2265.2008.03248.x18363885 [Google Scholar] [CrossRef] [PubMed]
[11]. Shoelson SE, Lee J, Goldfine AB, Inflammation and insulin resistanceJ Clin Invest 2006 116(7):1793-801.10.1172/JCI2906916823477 [Google Scholar] [CrossRef] [PubMed]
[12]. Juge-Aubry CE, Henrichot E, Meier CA, Adipose tissue: a regulator of inflammationBest Pract Res Clin Endocrinol Metab 2005 19(4):547-66.10.1016/j.beem.2005.07.00916311216 [Google Scholar] [CrossRef] [PubMed]
[13]. Pagano C, Pilon C, Olivieri M, Mason P, Fabris R, Serra R, Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humansJ Clin Endocrinol Metab 2006 91(8):3165-70.10.1210/jc.2006-036116720654 [Google Scholar] [CrossRef] [PubMed]
[14]. Haider DG, Schindler K, Schaller G, Prager G, Wolzt M, Ludvik B, Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric bandingJ Clin Endocrinol Metab 2006 91(4):1578-81.10.1210/jc.2005-224816449335 [Google Scholar] [CrossRef] [PubMed]
[15]. Vincent HK, Powers SK, Dirks AJ, Scarpace PJ, Mechanism for obesity-induced increase in myocardial lipid peroxidationInt J Obes Relat Metab Disord 2001 25(3):378-88.10.1038/sj.ijo.080153611319636 [Google Scholar] [CrossRef] [PubMed]
[16]. Kobayasi R, Akamine EH, Davel AP, Rodrigues MAM, Carvalho CRO, Rossoni L V, Oxidative stress and inflammatory mediators contribute to endothelial dysfunction in high-fat diet-induced obesity in miceJ Hypertens 2010 28(10):2111-9.10.1097/HJH.0b013e32833ca68c20616756 [Google Scholar] [CrossRef] [PubMed]
[17]. Olusi SO, Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humansInt J Obes Relat Metab Disord 2002 26(9):1159-64.10.1038/sj.ijo.080206612187391 [Google Scholar] [CrossRef] [PubMed]
[18]. Clarke MW, Burnett JR, Croft KD, Vitamin E in human health and diseaseCrit Rev Clin Lab Sci 2008 45(5):417-50.10.1080/1040836080211862518712629 [Google Scholar] [CrossRef] [PubMed]
[19]. Garcia-Bailo B, El-Sohemy A, Haddad PS, Arora P, BenZaied F, Karmali M, Vitamins D, C, and E in the prevention of type 2 diabetes mellitus: modulation of inflammation and oxidative stressBiol targets Ther 2011 5:7 [Google Scholar]
[20]. Asayama K, Kooy NW, Burr IM, Effect of vitamin E deficiency and selenium deficiency on insulin secretory reserve and free radical scavenging systems in islets: decrease of islet manganosuperoxide dismutaseJ Lab Clin Med 1986 107(5):453-58. [Google Scholar]
[21]. Manning PJ, Sutherland WHF, Walker RJ, Williams SM, De Jong SA, Ryalls AR, Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjectsDiabetes Care 2004 27(9):2166-71.10.2337/diacare.27.9.216615333479 [Google Scholar] [CrossRef] [PubMed]
[22]. Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart StudyCirculation 2007 116(11):1234-41.10.1161/CIRCULATIONAHA.107.71050917709633 [Google Scholar] [CrossRef] [PubMed]
[23]. Ayala A, Muñoz MF, Argüelles S, Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenalOxid Med Cell Longev 2014 :36043810.1155/2014/36043824999379 [Google Scholar] [CrossRef] [PubMed]
[24]. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Diagnosis and management of the metabolic syndromeCirculation 2005 112(17):2735-52.10.1161/CIRCULATIONAHA.105.16940416157765 [Google Scholar] [CrossRef] [PubMed]
[25]. Zaki M, Kamal S, Ezzat W, Hassan N, Yousef W, Ryad H, Serum apelin levels and metabolic risk markers in obese womenJ Genet Eng Biotechnol 2017 15(2)10.1016/j.jgeb.2017.05.00230647682 [Google Scholar] [CrossRef] [PubMed]
[26]. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in manDiabetologia 1985 28(7):412-19.10.1007/BF002808833899825 [Google Scholar] [CrossRef] [PubMed]
[27]. Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H, Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomesSteroids 1994 59(6):383-8.10.1016/0039-128X(94)90006-X [Google Scholar] [CrossRef]
[28]. Erel O, A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cationClin Biochem 2004 37(4):277-85.10.1016/j.clinbiochem.2003.11.01515003729 [Google Scholar] [CrossRef] [PubMed]
[29]. Jain SK, Levine SN, Duett J, Hollier B, Reduced vitamin E and increased lipofuscin products in erythrocytes of diabetic ratsDiabetes 1991 40(10):1241-4.10.2337/diab.40.10.12411936587 [Google Scholar] [CrossRef] [PubMed]
[30]. Blasiak J, Gloc E, Drzewoski J, Wozniak K, Zadrozny M, Skórski T, Free radical scavengers can differentially modulate the genotoxicity of amsacrine in normal and cancer cellsMutat Res Toxicol Environ Mutagen 2003 535(1):25-34.10.1016/S1383-5718(02)00289-9 [Google Scholar] [CrossRef]
[31]. Zaki M, Kamal S, Basha WA, El-Toukhy S, Yousef W, El-Bassyouni HT, Assessment of DNA damage in obese premenopausal women with metabolic syndromeGene Reports 2018 10:42-46.10.1016/j.genrep.2017.10.012 [Google Scholar] [CrossRef]
[32]. Anwar GM, Motawei AA, Ibrahim A, Galal A, Salama HM, Aly AA, Serum visfatin level in obese Egyptian children and adolescents and its relation with metabolic syndromeMed Res J 2015 14(2):53-58.10.1097/01.MJX.0000472995.93483.08 [Google Scholar] [CrossRef]
[33]. Hilali N, Kocyigit A, Demir M, Camuzcuoglu A, Incebiyik A, Camuzcuoglu H, DNA damage and oxidative stress in patients with mild preeclampsia and offspringEur J Obstet Gynecol Reprod Biol 2013 170(2):377-80.10.1016/j.ejogrb.2013.07.03123953912 [Google Scholar] [CrossRef] [PubMed]
[34]. Fasshauer M, Blüher M, Stumvoll M, Tönessen P, Faber R, Stepan H, Differential regulation of visfatin and adiponectin in pregnancies with normal and abnormal placental functionClin Endocrinol (Oxf) 2007 66(3):434-39.10.1111/j.1365-2265.2007.02751.x17302880 [Google Scholar] [CrossRef] [PubMed]
[35]. Fasshauer M, Waldeyer T, Seeger J, Schrey S, Ebert T, Kratzsch J, Serum levels of the adipokine visfatin are increased in pre-eclampsiaClin Endocrinol (Oxf) 2008 69(1):69-73.10.1111/j.1365-2265.2007.03147.x18034779 [Google Scholar] [CrossRef] [PubMed]
[36]. Kim JA, Choi YS, Hong JI, Kim SH, Jung HH, Kim SM, Association of metabolic syndrome with white blood cell subtype and red blood cellsEndocr J 2006 53(1):133-39.10.1507/endocrj.53.1331654368310.1507/endocrj.53.13316543683 [Google Scholar] [CrossRef] [PubMed] [CrossRef] [PubMed]
[37]. Filippatos TD, Derdemezis CS, Gazi IF, Lagos K, Kiortsis DN, Tselepis AD, Increased plasma visfatin levels in subjects with the metabolic syndromeEur J Clin Invest 2008 38(1):71-72.10.1111/j.1365-2362.2007.01904.x18173555 [Google Scholar] [CrossRef] [PubMed]
[38]. Sandeep S, Velmurugan K, Deepa R, Mohan V, Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian IndiansMetabolism 2007 56(4):565-70.10.1016/j.metabol.2006.12.00517379018 [Google Scholar] [CrossRef] [PubMed]
[39]. Esposito K, Ciotola M, Schisano B, Misso L, Giannetti G, Ceriello A, Oxidative stress in the metabolic syndromeJ Endocrinol Invest 2006 29(9):791-95.10.1007/BF0334737217114909 [Google Scholar] [CrossRef] [PubMed]
[40]. Ceriello A, Motz E, Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisitedArterioscler Thromb Vasc Biol 2004 24(5):816-23.10.1161/01.ATV.0000122852.22604.7814976002 [Google Scholar] [CrossRef] [PubMed]
[41]. Kim J, Wei Y, Sowers JR, Role of mitochondrial dysfunction in insulin resistanceCirc Res 2008 102(4):401-14.10.1161/CIRCRESAHA.107.16547218309108 [Google Scholar] [CrossRef] [PubMed]
[42]. Francisqueti FV, Chiaverini LCT, Santos KC dos, Minatel IO, Ronchi CB, Ferron AJT, The role of oxidative stress on the pathophysiology of metabolic syndromeRev Assoc Med Bras 2017 63(1):85-91.10.1590/1806-9282.63.01.8528225880 [Google Scholar] [CrossRef] [PubMed]
[43]. Tiganis T, Reactive oxygen species and insulin resistance: the good, the bad and the uglyTrends Pharmacol Sci 2011 32(2):82-89.10.1016/j.tips.2010.11.00621159388 [Google Scholar] [CrossRef] [PubMed]
[44]. Delavar R, Mogharnasi M, Khoobkhahi N, The Effects of Combined Training on Oxidative Stress and Antioxidant Defense IndicatorsInt J Basic Sci Med 2017 2(1):29-32.10.15171/ijbsm.2017.07 [Google Scholar] [CrossRef]
[45]. Ganjifrockwala FA, Joseph JT, George G, Decreased total antioxidant levels and increased oxidative stress in South African type 2 diabetes mellitus patientsJ Endocrinol Metab Diabetes South Africa 2017 22(2):21-25.10.1080/16089677.2017.1324590 [Google Scholar] [CrossRef]
[46]. Evans JL, Antioxidants: do they have a role in the treatment of insulin resistance?Indian J Med Res 2007 125(3):35510.12968/denn.2007.3.3.29682 [Google Scholar] [CrossRef]
[47]. Zahorska-Markiewicz B, Olszanecka-Glinianowicz M, Janowska J, Kocełak P, Semik-Grabarczyk E, Holecki M, Serum concentration of visfatin in obese womenMetabolism 2007 56(8):1131-34.10.1016/j.metabol.2007.04.00717618961 [Google Scholar] [CrossRef] [PubMed]
[48]. Villalobos LA, Uryga A, Romacho T, Leivas A, Sánchez-Ferrer CF, Erusalimsky JD, Visfatin/Nampt induces telomere damage and senescence in human endothelial cellsInt J Cardiol 2014 175(3):573-75.10.1016/j.ijcard.2014.05.02824874905 [Google Scholar] [CrossRef] [PubMed]
[49]. Garten A, Petzold S, Schuster S, Körner A, Kratzsch J, Kiess W, Nampt and its potential role in inflammation and type 2 diabetesIn: Diabetes-Perspectives in Drug Therapy 2011 Springer:147-64.10.1007/978-3-642-17214-4_721484571 [Google Scholar] [CrossRef] [PubMed]
[50]. Chen M-P, Chung F-M, Chang D-M, Tsai JC-R, Huang H-F, Shin S-J, Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitusJ Clin Endocrinol Metab 2006 91(1):295-99.10.1210/jc.2005-147516234302 [Google Scholar] [CrossRef] [PubMed]
[51]. Shaker O, El-Shehaby A, Zakaria A, Mostafa N, Talaat S, Katsiki N, Plasma visfatin and retinol binding protein-4 levels in patients with type 2 diabetes mellitus and their relationship to adiposity and fatty liverClin Biochem 2011 44(17):1457-63.10.1016/j.clinbiochem.2011.08.114821939650 [Google Scholar] [CrossRef] [PubMed]
[52]. Brentano F, Schorr O, Ospelt C, Stanczyk J, Gay RE, Gay S, Pre-B cell colony-enhancing factor/visfatin, a new marker of inflammation in rheumatoid arthritis with proinflammatory and matrix-degrading activitiesArthritis Rheumatol 2007 56(9):2829-39.10.1002/art.2283317763446 [Google Scholar] [CrossRef] [PubMed]