Introduction
Acromegaly is a clinical syndrome resulting from excessive secretion of growth hormone with the approximate prevalence of six cases per million. Somatostatin agonists are the only drug approved which can actually control, to some extent, the secretion of growth hormone after surgery. Short-acting analogues of these compounds should be taken three times a day which is very undesirable. In contrast, long-acting analogues of somatostatin have the market availability for more than one decade, but Iranian physicians have the drug accessibility only within the last few years.
Aim
To determine the effects of long-acting analogue octreotide (Sandostatin Long Acting Release (LAR) on the level of Growth Hormone (GH), Insulin-like Growth Factor 1 (IGF-1) and clinical symptoms in patients with acromegaly in the northwest of Iran.
Materials and Methods
The level of GH, IGF-1 and clinical symptoms were evaluated after long-acting analogue Sandostatin LAR administration in 40 acromegalic patients with no surgical history or any remarkable improvement in clinical features and IGF-1/GH levels after surgery, referred to outpatient endocrinology sections, Tabriz University of Medical Sciences were included. The serum level of GH and IGF-1 were evaluated using electro-chemiluminescence system before and after 3, 6 and 12-months of drug administration. Repeated measure test was used for statistical analysis. p<0.05 was considered as statistically significant.
Results
Use of Sandostatin LAR showed 72.5% and 27.5% absolute and partial recovery, respectively. Mean level of IGF-1 before and after 3, 6 and 12-months treatment were 654.17±199.12, 423.95±228.94, 356.22±169.53, 296.25±110.6 μg/L, respectively, (p<0.001). Additionally, Mean GH level before and after 3, 6 and 12-months treatment were 31.71±3.03, 10.31±5.23, 7.28±3.11 and 6.77±4.38 μg/L, respectively, (p<0.001).
Conclusion
Sandostatin LAR treatment may be an effective and proper method for treatment of GH producing adenoma in patients who are not good candidates for surgery or having high GH/ IGF-1 levels after surgery.
Adenoma, Growth hormone, Octreotide, Somatostatin agonists
Introduction
Acromegaly is a clinical syndrome resulting from excessive secretion of Growth Hormone (GH) with the approximate prevalence range between 2.8-13.7 cases per 100000 people [1]. The mean age at diagnosis is around 40-45 years. The most common cause of acromegaly is somatotroph secreting adenoma from anterior pituitary. The clinical symptoms of acromegaly are mainly due to the secretion of GH and IGF-1 [1]. It has been reported that high levels of these two hormones cause somatic and metabolic symptoms. However, somatotroph adenomas can cause mass effects [1].
Increased long-term secretion of these hormones causes excessive growth of many tissues, including connective tissue, cartilage, bone, skin and visceral organs. Other systemic complications include cardiovascular disorders, sleep apnea, metabolic diseases and colon cancer [2].
Diagnostic approach of this disease is the measurement of GH level and Glucose Tolerance Test (GTT) and is treated by reduction of pituitary mass and suppression or inhibition of GH and IGF-1 secretion [3].
There is a general consensus that preferred treatment for GH secreting adenomas is trans-sphenoid surgery. However, the surgical treatment is curative only in 50.6% of patients [4]. Additionally, some patients with comorbidities are not candidates or refuse the surgery [4].
Other treatment approaches include medical, and radiation based therapies. Although, the satisfactory outcomes of radio-therapy usually require a long-time period to be achieved. There are three categories of medication including somatostatin analogues, dopamine agonist, and GH receptor antagonist. Somatostatin analogues are usually well tolerated by patients and the main side effect is gastrointestinal complications [5,6].
The results of treatment with somatostatin are different in various studies. For instance, regarding Somatostatin Receptors (SSTRs) phenotype, higher gene expression ratio of SSTR2/SSTR5 has been associated with better control of hormonal disorders in somatotropinomas in response to Sandostatin LAR [7]. Additionally, SSTR mutations have also been linked to influence the Sandostatin LAR responsiveness, so that, SSTR5 Arg240Trp mutation is correlated to impaired anti-secretory effects and complete resistance to the anti-proliferative effect of Sandostatine LAR [8]. Therefore, it seems that several factors, including genetic backgrounds, are involved in treatment efficacy [9].
At the time of the write-up of this study, no study has been conducted on the clinical and laboratory outcomes of Sandostatin LAR treated acromegalic patients in northwest of Iran. As regards, somatostatin analogues, particularly Sandostatin LAR is currently available in Iran and many acromegalic patients are taking these agents for more than one year, and there is significant lack of information on its effectiveness. Thus, the present authors planned the present study to determine the effects of Sandostatin LAR on the level of GH, IGF-1 and clinical symptoms in patients with acromegaly.
Materials and Methods
Patients
In this retrospective study, 40 acromegalic patients, with no surgical history and those with no remarkable improvement in clinical features and IGF-1/GH levels after surgery, referred to outpatient endocrinology sections, Tabriz University of Medical Sciences, during the period of June 2015 to June 2016 were included. All available acromegaly patients treated with 20 mg/month Intramuscular injection (IM) of Sandostatin LAR. Good surgery outcomes and refusal to take medication during last one year for any reason were also considered as exclusion criteria.
Individuals’ information including clinical symptoms and diagnostic methods were extracted from the patients’ records. The initiation date of therapy, patient’s compliance with drug and the number of Sandostatin shots injected in a year were also recorded in a questionnaire. Based on patients’ declarations, the subjective (sweating, physical strength, sleep status, joint pains, paresthesia, back pain, skin changes, and sleep apnea) and the objective (great extremities, condition of the scalp and face, nose, teeth, symptoms of carpal tunnel syndrome, hypertension) symptoms were extracted from patients medical documents after treatment. However, because of retrospective design of this study, full access to all data was not achievable.
Improvement in clinical symptoms including the headache, nausea, strabismus and reduction in IGF-1 and GH levels were also considered as partial/absolute recovery among patients.
Laboratory Findings
All laboratory tests result ordered at baseline and 3, 6 and 12-months after drug administration were extracted from patients’ documents. GH and IGF-1 had been evaluated using an electro-chemiluminescence (Hitachi e411) system.
Ethics
This study was approved by the Research and Ethics Committees of Tabriz University of Medical Sciences (code: 93/3-10/14).
Statistical Analysis
Results were expressed as mean±Standard Deviation (SD). SPSS 23.0 software (SPSS, Inc., Chicago, IL) was used for data analysis. The statistical significance of the results was determined using repeated measure test. p<0.05 was considered as statistically significant.
Results
The total numbers of subjects with usable and accessible data were 40 out of 58 patients (21 male and 19 female). Three patients had no surgical history while 27 and 10 patients underwent one or two surgery, respectively. The average age of male and female patients was 43.71±13.36 and 44.15±13.76 years, respectively. General characteristics of patients are shown in [Table/Fig-1].
General characteristics and clinical symptoms after 12 months treatment in acromegalic patients.
| Patients (n=40) |
|---|
| Variables | Male Number (%) | Female Number (%) |
|---|
| Sex | 21 (52.5) | 19 (47.5) |
| Age (year) | 43.71±13.36 | 44.15±13.76 |
| History of surgery | None | 1 (4.7) | 2 (10.5) |
| Once | 15 (71.4) | 12 (63.1) |
| Twice | 5 (23.8) | 5 (26.3) |
| History of radiotherapy | 4 (19.04) | 4 (21.05) |
| Clinical symptoms |
| Headache | 21 (100) | 19 (100) |
| Nausea | 7 (33.3) | 3 (15.7) |
| Strabismus | 15 (71.4) | 12 (63.1) |
Data are presented as number, % of total and mean±SD
As shown in [Table/Fig-2], based on patients’ declarations, full and partial recovery in the subjective and the objective symptoms which were explained above were achieved in 72.5% (71.4% males and 73.6% females) and 27.5% (28.6% males and 26.4 females) of patients, respectively.
Full and partial recovery of patients after long-acting octreotide administration.
| N=40 |
|---|
| Full recovery Number (%) | Partial recovery Number (%) |
|---|
| Recovery | 29 (72.5) | 11 (27.5) |
| Male | Female | Male | Female |
| 15 (51.72) | 14 (48.28) | 6 (54.54) | 5 (45.46) |
Data are presented as number and % of total.
Fully recovered subjects were patients having no subjective symptoms and normal IGF-1 during study period.
Partially recoveredsubjects were patients having above normal IGF-1 than pretreatment levels and/or with subjective symptoms
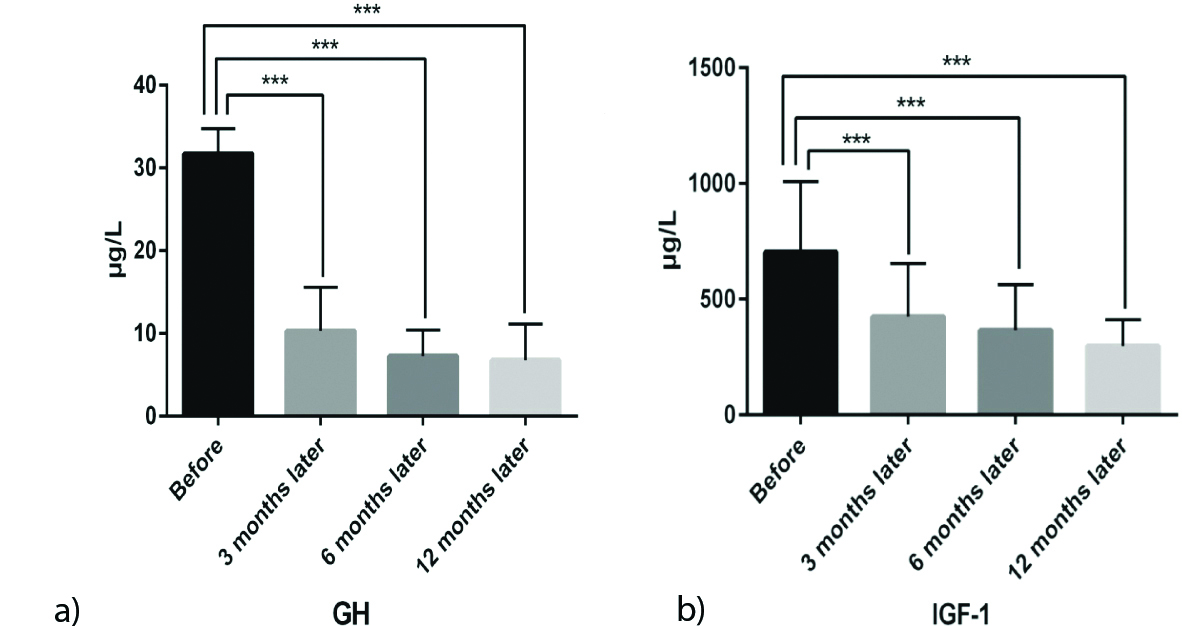
Mean level of IGF-1 before and after 3, 6 and 12-months treatment were 654.17±199.12, 423.95±228.94, 356.22±169.53, 296.25±110.6 μg/L, respectively, (p<0.001) [Table/Fig-3a]. Additionally, Mean GH level before and after 3, 6 and 12 months treatment were 31.71±3.03, 10.31±5.23, 7.28±3.11 and 6.77±4.38 μg/L, respectively, (p<0.001) [Table/Fig-3b].
Mean levels of serum GH (a). and IGF-1 (b). in different time periods.
(*** P<0.001). GH: growth hormone; IGF-1: insulin-like growth factor-1.
Discussion
The present data showed a significant reduction in serum IGF-1 and GH levels after 12-months administration of Sandostatin LAR indicating the effectiveness of this drug in acromegalic patients. Additionally, 72.5% and 27.5% of patients expressed absolute and partial recovery after this time period according to their clinical symptoms.
It has been reported that use of long-acting somatostatin analogues results in GH and IGF-1 reduction in 50-65% of cases. Also, using this medication in 65% of cases has normalised the level of IGF-1 [10]. Different studies have also evaluated the efficacy of octreotide and its long-acting products in the treatment of acromegaly [11,12]. This drug reduces GH in 90% of patients and in 40-50% of cases; GH levels have been reported to be decreased <10 μg/L [6]. Additionally, one side effect of this agent is impaired glucose tolerance, suggesting the necessity of long-term follow-up during this medication use [10]. However, in the present study, the status of glucose tolerance was not evaluated.
Continuous infusion of octreotide also suppresses GH for 24-hours [13]. Although the effect of Sandostatin LAR is similar to subcutaneous octreotide, it decreases the fasting level of GH to a greater extent [14]. Additionally, four-week intervals for prescribing Sandostatin LAR are recommended by the producer factory. Biermasz NR et al., also reported that single-shot injections of Sandostatin LAR in a six-week interval exhibit similar results as four-weeks interval regimen [15]. They found that the mean serum GH concentration became <5 mU/L in all patients and normal serum IGF-1 was observed in 11 out of 14 patients at 26-weeks and 9 out of 13 patients at 44 weeks.
Colao A et al., also revealed an improvement in GH, IGF-1 and tumour size in 36 acromegalic patients after Sandostatin LAR administration in 12-24 months period intervals [16]. Normal levels of GH and IGF-1 were observed in 9.4% and 61% of patients, respectively. A medium to high reduction in tumour size was also reported in 53.3% of patients who had received initial medical therapy.
Cozzi R et al., also showed similar results with a 68.7%, 70.1% and 82.1% improvement in GH, IGF-1 and tumour size in 67 acromegalic patients, respectively, after prescribing Sandostatin LAR and 48-month follow-up [17].
Furthermore, joints thickness reduction is observed after corrected GH and IGF-1 levels following administration of Sandostatin LAR indicating improvement of tissue hypertrophies in acromegalic patients [18].
Disease symptoms such as the severe headache, sweating, joint pain, swelling of the extremities and weakness have also been reported to be significantly reduced or completely eliminated [19]. However, exact accessibility to all these data was not possible in the present study.
Limitation
The present study showed an improvement in serum GH and IGF-1 levels and clinical features after Sandostatin LAR administration in acromegaly patients. However, higher numbers of patients and evaluation of glucose tolerance could result in a better data interpretation for a more comprehensive evaluation of drug efficacy. Additionally, analysis of genetic variations account for Sandostatin LAR cellular response may help accurate and targeted drug administration regarding to personalised medicine.
Conclusion
The present data indicates an overall improvement in clinical symptoms including severe headache, sweating, joint pain, swelling of the extremities and weakness and the level of IGF-1 and GH in acromegalic patients following use of long-acting octreotide and this drug may be considered as an effective method for treatment of acromegaly in patients who are poor candidates for surgery.
Data are presented as number, % of total and mean±SD
Data are presented as number and % of total.
Fully recovered subjects were patients having no subjective symptoms and normal IGF-1 during study period.
Partially recoveredsubjects were patients having above normal IGF-1 than pretreatment levels and/or with subjective symptoms
[1]. Lavrentaki A, Paluzzi A, Wass JA, Karavitaki N, Epidemiology of acromegaly: review of population studiesPituitary 2017 20(1):4-9.10.1007/s11102-016-0754-x27743174 [Google Scholar] [CrossRef] [PubMed]
[2]. Molitch M, Clinical manifestations of acromegalyEndocrinolMetabClin North Am 1992 21(3):597-614.10.1016/S0889-8529(18)30204-4 [Google Scholar] [CrossRef]
[3]. Giustina A, Barkan A, Casanueva FF, Cavagnini F, Frohman L, Ho K, Criteria for cure of acromegaly: a consensus statementJ Clin Endocrinol Metab 2000 85(2):526-29.10.1210/jcem.85.2.636310690849 [Google Scholar] [CrossRef] [PubMed]
[4]. Losa M, Mortini P, Barzaghi R, Ribotto P, Terreni MR, Marzoli SB, Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrenceJ Neurosurg 2008 108(3):525-32.10.3171/JNS/2008/108/3/052518312100 [Google Scholar] [CrossRef] [PubMed]
[5]. Manjila S, Wu OC, Khan FR, Khan MM, Arafah BM, Selman WR, Pharmacological management of acromegaly: a current perspectiveNeurosurg Focus 2010 29(4):E1410.3171/2010.7.FOCUS1016820887124 [Google Scholar] [CrossRef] [PubMed]
[6]. Vance ML, Harris AG, Long-term treatment of 189 acromegalic patients with the somatostatin analog octreotide: results of the International Multicenter Acromegaly Study GroupArch Intern Med 1991 151(8):1573-78.10.1001/archinte.1991.00400080073013 [Google Scholar] [CrossRef]
[7]. Taboada GF, Luque RM, Neto LV, Guimaraes RFC, Marcondes JB, Chimelli LMC, Quantitative analysis of somatostatin receptor subtypes (1-5) gene expression levels in somatotropinomas and correlation to in vivo hormonal and tumor volume responses to treatment with octreotide LAREur J Endocrinol 2008 158:295-303.doi: 10.1530/EJE-07-056210.1530/EJE-07-056218299461 [Google Scholar] [CrossRef] [PubMed]
[8]. Ballare E, Persani L, Lania AG, Filopanti M, Giammona E, Corbetta S, Mutation of somatostatin receptor type 5 in an acromegalic patient resistant to somatostatin analog treatmentJ Clin Endocrinol Metab 2001 86:3809-14.doi: 10.1210/jcem.86.8.778710.1210/jcem.86.8.778711502816 [Google Scholar] [CrossRef] [PubMed]
[9]. Filopanti M, Ronchi C, Ballare E, Bondioni S, Lania A, Losa M, Analysis of somatostatin receptors 2 and 5 polymorphisms in patients with acromegalyJ Clin Endocrinol Metab 2005 90(8):4824-28.doi: 10.1210/jc.2005-013210.1210/jc.2005-013215914528 [Google Scholar] [CrossRef] [PubMed]
[10]. Ayuk J, Stewart SE, Stewart PM, Sheppard MC, Long-term safety and efficacy of depot long-acting somatostatin analogs for the treatment of acromegalyJ ClinEndocrinolMetab 2002 87(9):4142-46.10.1210/jc.2001-01191312213862 [Google Scholar] [CrossRef] [PubMed]
[11]. Grunstein RR, Ho KK, Sullivan CE, Effect of octreotide, a somatostatin analog, on sleep apnea in patients with acromegalyAnn Intern Med 1994 121(7):478-83.10.7326/0003-4819-121-7-199410010-000028067645 [Google Scholar] [CrossRef] [PubMed]
[12]. Bevan J, Atkin S, Atkinson A, Bouloux PM, Hanna F, Harris P, Primary medical therapy for acromegaly: an open, prospective, multicenter study of the effects of subcutaneous and intramuscular slow-release octreotide on growth hormone, insulin-like growth factor-I, and tumor sizeJ Clin Endocrinol Metab 2002 87(10):4554-63.10.1210/jc.2001-01201212364434 [Google Scholar] [CrossRef] [PubMed]
[13]. Tauber J, Babin T, Tauber M, Vigoni F, Bonafe A, Ducasse M, Long term effects of continuous subcutaneous infusion of the somatostatin analog octreotide in the treatment of acromegalyJ Clin Endocrinol Metab 1989 68(5):917-24.10.1210/jcem-68-5-9172565913 [Google Scholar] [CrossRef] [PubMed]
[14]. Hunter S, Shaw J, Lee K, Wood P, Atkinson A, Bevan J, Comparison of monthly intramuscular injections of Sandostatin LAR with multiple subcutaneous injections of octreotide in the treatment of acromegaly; effects on growth hormone and other markers of growth hormone secretionClin Endocrinol (Oxf) 1999 50:245-52.10.1046/j.1365-2265.1999.00668.x10396369 [Google Scholar] [CrossRef] [PubMed]
[15]. Biermasz NR, Van Den Oever NC, Frölich M, Arias AMP, Smit JW, Romijn JA, Sandostatin LAR in acromegaly: a 6-week injection interval suppresses GH secretion as effectively as a 4-week intervalClin Endocrinol 2003 58(3):288-95.10.1046/j.1365-2265.2003.01710.x12608933 [Google Scholar] [CrossRef] [PubMed]
[16]. Colao A, Ferone D, Marzullo P, Cappabianca P, Cirillo S, Boerlin V, Long-term effects of depot long-acting somatostatin analog octreotide on hormone levels and tumor mass in acromegalyJ Clin Endocrinol Metab 2001 86(6):2779-86.10.1210/jcem.86.6.755611397887 [Google Scholar] [CrossRef] [PubMed]
[17]. Cozzi R, Montini M, Attanasio R, Albizzi M, Lasio G, Lodrini S, Primary treatment of acromegaly with octreotide LAR: a long-term (up to nine years) prospective study of its efficacy in the control of disease activity and tumor shrinkageJ Clin Endocrinol Metab 2006 91(4):1397-403.10.1210/jc.2005-234716449332 [Google Scholar] [CrossRef] [PubMed]
[18]. Colao A, Cannavo S, Marzullo P, Pivonello R, Squadrito S, Vallone G, Twelve months of treatment with octreotide-LAR reduces joint thickness in acromegalyEur J Endocrinol 2003 148(1):31-38.10.1530/eje.0.148003112534355 [Google Scholar] [CrossRef] [PubMed]
[19]. Szücs N, Meszaros J, Czirjak S, Mondok A, Varga I, Glaz E, Experience in treating acromegalic patients with long-acting octreotideOrvHetil 2002 143(19 Suppl):1066-70. [Google Scholar]