The term ASB is used to describe a state in which bacteria are present in significant counts in the urine of a person with no apparent symptoms of UTI [1]. The association of ASB with adverse outcomes of pregnancy is a matter of concern. It can lead to symptomatic UTI in pregnant women, which could have devastating effects on both maternal and fetal health [2]. Serious consequences of ASB in pregnant women include pyelonephritis, preterm delivery, low birth weight and fetal death [3]. However, some studies have also questioned the ASB’s impact on adverse obstetric outcome. Nonetheless, there is unanimity on a greater risk of pyelonephritis [4]. The prevalence of ASB in pregnancy is estimated to be between 1.9-15% [5]. Most studies, including those from developing countries have reported similar rates [6]. Interestingly, some studies from Nigeria have reported higher rates [7,8]. ASB is reported to be related to socioeconomic status [9]. Recurrent UTI and Diabetes Mellitus (DM) as well, have been shown to be associated with ASB [10]. However, other recent studies have shown no significant difference in prevalence of ASB among pregnant women with DM or Gestational DM (GDM) and those without DM or GDM [11]. Association of other factors such as age, parity and race are ambiguous [6].
Timing for screening of ASB in pregnancy had been a matter of debate. Infectious Disease Society of America recommends that screening for ASB should be done at least once in early pregnancy and if found positive, should be treated with antibiotics. Periodic screening is advised subsequently for such women [1]. Screening during the first prenatal visit is recommended by US preventive services task force [11]. Several other national and international health agencies have included similar guidelines [2]. A study has shown that screening before 20 weeks of gestation could miss more than half of the case [12]. Other studies have shown that around 10-20% of pyelonephritis occur in first trimester [13]. A large meta-analysis of randomised clinical trials of ASB in pregnancy has demonstrated the beneficial effect of antibiotic treatment in decreasing the risk of development of pyelonephritis [14].
Cultural, medical, socioeconomic and personal hygienic factors reportedly influence the prevalence rates. Whalley P has described a higher rate among women attending public health hospitals compared to those attending the private ones [9]. Recurrent UTI, pre-pregnancy history of UTI, diabetes, and anatomical abnormalities of the urinary tract are postulated to be other risk factors [2].
Studies from the Arabian Gulf region indicate the prevalence rates from 3.3-13% [15-18]. Few studies have been conducted in Saudi Arabia to investigate ASB in pregnancy [19-21], most of such studies are of several years or even decades old. Al-Sibai MH et al., has reported prevalence rate of 14.2% in the Eastern Province of Saudi Arabia previously [20]. This issue needed a relook to assess the current extent of the problem. The objectives of this study were; to determine the current prevalence, measure the trend and investigate the association of socio-demographic, medical and obstetric characteristics of ASB among pregnant women in Eastern Saudi Arabia.
Materials and Methods
Study Design and Setting
A descriptive cross-sectional study was conducted in Al-Ahsa and Al-Khobar in the Eastern Province of Saudi Arabia, from June 2016 to December 2016. Approval for the study was taken from the Institutional Research Ethics Committee of College of Medicine, King Faisal University (Research code 14-11-18-B). Three hospitals were enrolled in the study; Maternity and Children Hospital, Al-Ahsa, King Fahad Hospital of the University and Al-Moosa Hospital, Al-Ahsa. Pregnant women attending routine antenatal checkups were included in the study. The subjects were informed about the study; assured of complete confidentiality and consent was obtained to participate in the study. A structured questionnaire was used to collect socio-demographic, obstetric and medical information. Gestational age was calculated from the first day of the last menstrual period.
Sample Size
The sample size was calculated for one group with a 99% confidence level, 5% margin of error and the prevalence proportion of the study condition as 0.15 from previous studies. The expected power of the study was 80%. The estimated sample size was 338 and additional subjects were included to make adjustments for effect size.
Inclusion Criteria
Pregnant women attending regular antenatal care and who consented to participate in the study were randomly included.
Exclusion Criteria
Women who declined to participate in the study, who reported with symptoms of UTI and who were taking antibiotics or had received antibiotics in the past two weeks: were excluded from the study.
Urine Collection and Processing
The study participants were explained about proper collection method of urine and were provided sterile container to collect two consecutive clean catch, midstream urine samples. The urine samples were transported and processed in the laboratory within an hour. Semiquantitative standard loop culture method was used for estimating the urine bacterial counts. Uncentrifuged 0.001 mL urine was inoculated on blood agar and Cysteine, Lactose, and Electrolyte Deficient Agar (CLED agar) and the plates were incubated at 37°C for 24 hours. Growth of a single potential uropathogens (such as any Gram negative bacilli, Staphylococcus spp., and Enterococcus spp.) or Streptococcus agalactiae (Group B Streptococcus or GBS) in a count ≥105, was considered as significant bacteriuria. Any mixed growth of skin or urogenital microbiota (such as Corynebacterium spp., Neisseria spp. Lactobacillus spp. Viridans Streptococci, coagulase negative Staphylococci) was disregarded as contamination. Oxidase test was done to differentiate members of the Enterobacteriaceae family from other Gram negative bacilli. The biochemical tests used for identification of Enterobacteriaceae family included Indole, Methyl Red, Voges-Proskeur, Citrate, Urease and hydrogen sulphide production tests [22]. Suspected colonies of Streptococcus spp., Staphylococcus spp. and Enterococcus spp. were initially identified by Gram staining and catalase test. Complete identification of such Gram positive cocci was done by VlTEK 2 Compact bacterial identification system (bioMérieux Inc., France). Antimicrobial sensitivity testing was done for the bacterial isolates by Kirby-Bauer’s disc diffusion. Antibiotic discs were obtained from Oxoid, United Kingdom. Discs recommended for urinary pathogens were tested. Testing and interpretation of the results was done according to Clinical Laboratory Standard Institute guidelines (2016) [23].
Statistical Analysis
Results were analysed using Statistical Package for Social Sciences (SPSS) IBM software version 22 (SPSS, Inc., Chicago, IL, USA). The qualitative data were calculated as the number and percentage and the quantitative data was mentioned in terms of mean, range and standard deviation. The Chi-square test was used to compare the categorical variables. The p-value of ≤0.05 was considered statistically significant.
Results
A total of 449 urine samples were screened from the three hospitals included in this study. Highest numbers of samples were from King Fahad University Hospital, Al-Khobar (n=334). The remaining 115 samples were from Maternity and Children Hospital and Al-Moosa Hospital (n=83 and 32, respectively). Significant bacterial growth on blood agar and CLED agar was seen in 64/449 urine samples (14.25%) [Table/Fig-1,2]. Six of the 449 samples (1.34%) grew Candida spp. The mean age of subjects was 30.38±5.84 years (range 20-46 years). Most of the subjects were aged 25-34 years (cumulative N=271). The majority of the women were in the third trimester of gestation (N=278) and the mean gestational age was 27.94±9.09 weeks (range 4-41 weeks). Most were multigravida with mean gravidity 3.39±2.42 (range 0-18). Fifteen women reported to be pregnant for more than 9 times [Table/Fig-3].
Significant bacterial growth on sheep blood agar.
Significant bacterial growth of lactose fermenter on CLED agar.
Characteristics of pregnant women with ASB.
| Characteristics | Number of subjects | Number of ASB (Percentage) |
|---|
| Maternal Age (Years) (N=449, Missing=0) |
| 18-24 | 72 | 10 (13.89) |
| 25-29 | 145 | 20 (13.8) |
| 30-34 | 126 | 21 (16.67) |
| 35-39 | 73 | 9 (12.33) |
| >40 | 33 | 4 (12.12) |
| History of UTI (N=449, Missing=0) |
| Yes | 88 | 16 (18.18) |
| No | 361 | 48 (13.30) |
| Gestational Age (N=447, Missing=2) |
| First Trimester (1-12 weeks) | 31 | 4 (12.12) |
| Second Trimester (13-27 weeks) | 138 | 17 (12.32) |
| Third Trimester (<=28 weeks) | 278 | 43 (15.47) |
| Gravidity (N=449, Missing=0) |
| Primi gravida | 105 | 11 (10.48) |
| 2-4 | 240 | 43 (17.92) |
| 5-8 | 89 | 8 (8.99) |
| 9-18 | 15 | 3 (25) |
| Maternal Medical Conditions |
| HTN | 20 | 4 (20) |
| DM | 21 | 2 (9.52) |
| GDM | 64 | 9 (14.06) |
The prevalence and association of different characteristics of pregnant women with ASB were also analysed using Chi-square test [Table/Fig-4]. It was highest among women aged 30-34 years (16.67%) and the p-value was found significant (0.05). Higher numbers of women in the third trimester of pregnancy were found with ASB (15.47%) and the p-value was significant (0.03). Pregnant women with past history of UTI had a significant association with ASB (p-value: 0.03). No statistical significance of maternal diabetes, GDM or Hypertension (HTN) was found with the ASB.
Association of patient characteristics with ASB.
| Variables | Chi-square | p-value |
|---|
| Maternal age | 18.24 | 0.05 |
| History of UTI | 20.2 | 0.03 |
| Gravidity | 60.7 | 0.2 |
| Gestational age | 37 | 0.03 |
| Maternal DM | 5.2 | 0.2 |
| GDM | 7.4 | 0.2 |
| Hypertension | 6.8 | 0.2 |
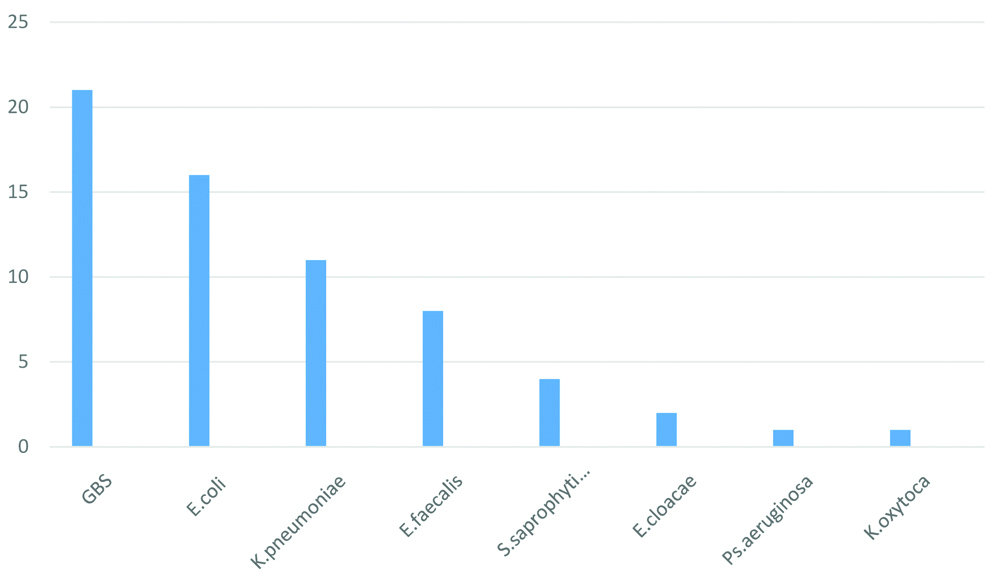
A range of biochemical tests were used to identify the Gram negative bacterial isolates [Table/Fig-5], as mentioned earlier. Of the 64 bacteria isolated in significant counts, Group B Streptococcus (GBS) was found to be the most common (N=21, 32.81%). However, among the known uropathogens, Escherichia coli was the commonest (N=16, 25%). Klebsiella pneumoniae was the second most frequent uropathogen (11, 17.18%) [Table/Fig-6].
Identification of Gram negative bacterial isolates by conventional biochemical tests.

Bacterial isolates of ASB (in numbers).
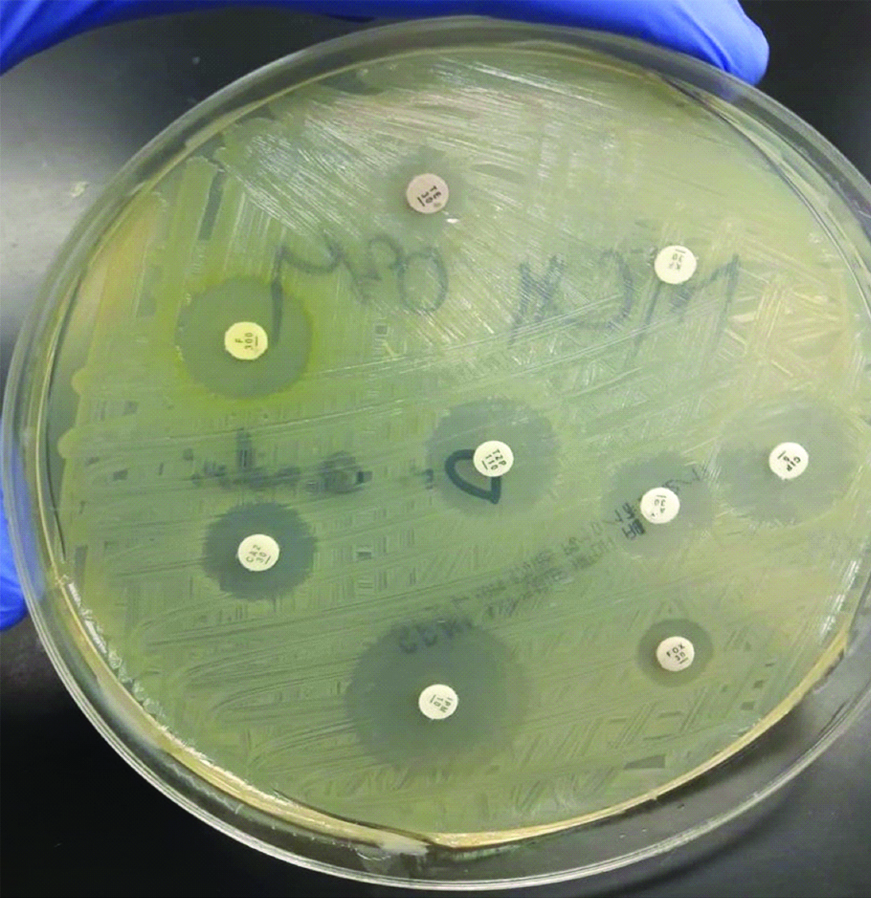
Kirby-Bauer’s disc diffusion test was done to determine antibiotic susceptibility of the bacterial isolates [Table/Fig-7]. The antibiotic sensitivity profile is shown in the [Table/Fig-8]. All the E. coli and K. pneumoniae isolates were sensitivity to Imipenem. High resistance was seen against ampicillin among all isolates. However, three fourth of the GBS isolates were sensitive to ampicillin. Sulfamethoxazole/Trimethoprim, a recommended drug for treating UTI, was in vitro effective against 50% and 45.5% of E.coli and K. pneumoniae isolates respectively. The majority of the Gram negative bacilli were sensitive to Cefoxitin, Piperacillin/Tazobactum, Ciprofloxacin and Ceftazidime.
Antibiotic susceptibility testing of bacterial isolate by Kirby Bauer disc diffusion method.
Antibiotic sensitivity profile of urinary bacterial isolates (Percentage sensitive).
| Antibiotics | GBS N=21 | E.coli N=16 | K. pneumoniae N=11 | E. feacalis N=8 | S.saprophyticus N=4 | E. cloacae N=2 | Ps. aeruginosa N=1 | K. oxytoca N=1 |
|---|
| No (%) | MIC* | No (%) | MIC$ | No (%) | No (%) | MIC | No (%) | MIC | No | No | MIC | No |
|---|
| AMP | 16 (76.2) | S≤0.25 | 2 (12.5) | S≤8 R≥32 | 2 (18) | 6 (75) | S≤8 R≥16 | 0 | S≤0.25 R≥0.5 | 0 | - | | 0 |
| AUG | - | - | 4 (25) | S≤8/4 R≥32/16 | 5 (45.5) | - | | 2 | S≤8/4R≥32/16 | 1 | - | | 1 |
| TET | 7 (33.5) | S≤4 R≥16 | 10 (62.5) | S≤4R≥16 | 9 (82) | 2 (25) | S≤4R≥16 | 3 | S≤4R≥16 | 2 | - | | 1 |
| SXT/TRI | 6 (28.6) | S≤2/38R≥4/76 | 8 (50) | S≤2/38R≥4/76 | 5 (45.5) | - | | 3 | S≤2/38 R≥4/76 | 0 | - | | 1 |
| CZN | - | - | 6 (37.5) | S≤16R≥32 | 5 (45.5) | - | | - | | 1 | - | | 1 |
| FOX | - | - | 12 (75) | S≤8R≥32 | 9 (82) | - | | - | | 1 | - | | 1 |
| GEN | 18 (85.7) | S≤4R ≥16 | 8 (50) | S≤4R≥16 | 10 (91) | 6 (75) | S≤500 R≥500 | 2 | S≤4R≥16 | 2 | 0 | S≤4 R≥16 | 1 |
| NF | - | - | 12 (75) | S≤32R≥128 | 10 (91) | 6 (75) | S≤32 R≥128 | 3 | S≤32 R≥128 | 2 | 1 | S≤32 R≥128 | 1 |
| PIP/TAZ | - | - | 14 (87.5) | S≤16/4R≥128/4 | 11 (100) | - | | - | | 2 | 0 | S≤16/4 R≥128/4 | 1 |
| OXA | - | - | - | | - | - | | 3 | S≤0.25 R≥0.5 | - | - | | - |
| VAN | 21 (100) | S≤1 | - | | - | 8 (100) | S≤4R≥32 | 4 | S≤4R≥ 32 | - | - | | - |
| MOX | - | - | - | | - | - | | 3 | S≤0.5 R≥2 | - | - | | - |
| CIP | - | - | 14 (87.5) | S≤1R≥4 | 10 (91) | 4 (50) | S≤1R≥4 | - | | 2 | 1 | S≤1R≥4 | 1 |
| IMI | - | - | 16 (100) | S≤1R≥4 | 11 (100) | - | | - | | 2 | 1 | S≤1R≥4 | 1 |
| CAZ | - | - | 14 (87.5) | S≤4R≥16 | 7 (64) | - | | - | | 1 | 1 | S≤4 R≥16 | 1 |
AMP: Ampicillin; AUG: Amoxicillin/clavulanic acid; TET: Tetracycline; SXT/TRI: Sulfamethoxazole/trimethoprim; CZN: Cefazolin; FOX: Cefoxitin; GEN: Gentamicin; NF: Nitrofurantoin; PIP/TAZ: Piperacillin/Tazocin; OXA: Oxacillin; VAN: Vancomycin; MOX: Moxifloxacin; CIP: Ciprofloxacin; IMI: Imipenem; CAZ: Ceftazidime
*All MIC values are in μg/ML $MIC values of E.coli are applicable to other Enterobacteriaceae spp.
Discussion
ASB in pregnancy is not uncommon. Its impact on pregnancy outcome has been a matter of debate. Pyelonephritis is shown to occur more often among pregnant women with ASB in comparison to those without it [4]. Several international organisations recommend detection and treatment of ASB during pregnancy [1,2,11].
The prevalence of ASB in this study was 14.25%. This is on a higher side than most other studies in recent years. Prevalence rates are reported to be between 1.9-15% [5] however, some studies from Sub-Saharan African countries have reported a considerably higher prevalence [7,8]. A noticeable feature of our finding is that a large number of isolates were GBS. GBS isolated from urine even in significant counts is rarely a cause of pyelonephritis [2]. Studies so far conducted in Saudi Arabia, though not many have reported highly varying prevalence rates. In 1989, Al-Sibai MH et al., found 14.2% subjects in their study with ASB. This study was also done in Eastern Province of Saudi Arabia and included 597 women [20] Our finding is similar to Al-Sibia MH et al., ASB among pregnant women is to the same extent as in the past, this part of Saudi Arabia. Abduljabbar H et al., in Jeddah found 7.1% of symptomless pregnant women with significant bacteriuria [19]. A recent retrospective study from Jeddah has documented relatively low prevalence of ASB in pregnancy (1.7%). The authors reviewed urine culture findings of 9,698 pregnant women attending routine antenatal clinic. Saudi subjects had relatively higher prevalence in comparison to Non-Saudis (2.3% vs. 0.7%) [21]. Other studies from adjoining Gulf countries have also reported different prevalence rates. In United Arab Emirates, Abdullah AA et al., detected 4.8% of pregnant women with ASB [15]. The prevalence of ASB in the countries of our region and beyond, reported is: Yemen 30% [24]. Egypt 10% [25], Pakistan 28.5% [26], Turkey 10.6% [27] and Iran 13% [18]. The only country with comparable socioeconomic and demographic characters with Saudi Arabia is the UAE. There are considerable variations in sample size in these studies which could have affected the results. A meta-analysis of 20 studies from Iran has reported 13% prevalence rate, nearer to our finding. The authors had classified studies into high, intermediate and low quality according to STROBE checklist. The prevalence of ASB in intermediate quality studies was 14%; greater than lower quality studies. Regional differences were also noticed in the study [18].
Higher occurrence of ASB in pregnancy is linked to lower socioeconomic status, history of recurrent UTI, diabetes mellitus and anatomical abnormalities of the urinary tract [2]. Poor personal hygiene and sexual activity are also cited as risks for bacteriuria [18]. Saudi Arabia has fairly well established public and private health care delivery systems. A relatively higher prevalence in this study could be due to several reasons. As most of the subjects were attending public hospitals, we assume that our sample was skewed towards the lower socioeconomic status and illiterate or semi-literate subjects. The per capita income of the participants was not taken because of socio-cultural reasons. Multiple gravidity is a known risk factor [28] and the number of multigravida women in our study was greater. Poor personal hygiene of women attending public hospitals could also be another reason. Unclear questioning, misunderstanding or personal inhibitions to reveal any urinary complaints could have also inadvertently included symptomatic cases. This might have influenced the results.
History of UTI in the past also increases the chances of ASB [2]. There was a significant difference between the occurrences of ASB among women with a past history of UTI against those without it (p-value: 0.03). A higher proportion of pregnant women in their third trimester had bacteriuria. Our results are in agreement with the findings of Al-Haddad AM et al., [24]. No association of medical conditions like DM, GDM, or hypertension was found with bacteriuria. These findings concur with earlier studies [11].
GBS was the most common bacteria isolated in our study (32.81%). GBS bacteriuria in pregnancy is considered a sign of heavy colonisation of the maternal genital tract [2,29]. GBS is an uncommon cause of UTI [2]. GBS colonisation of urinary tract was found to have significant association with premature rupture of membranes, preterm delivery, intrapartum fever and chorioamnionitis [2,6,29]. The preponderance of GBS bacteriuria in pregnancy, though not necessarily being the leading cause, is noted in other studies as well. E.coli was the second most common isolate in our study. Other studies have reported it as the most common isolate [15,19,25,26].
In this study imipenem, ciprofloxacin, piperacillin/tazobactum, cefoxitin, ceftazidime had a good sensitive profile against urinary isolates. Nitrofurantoin was an effective drug in vitro against both Gram negative and Gram positive bacteria, whereas sulfamethoxazole/trimethoprim was relatively more resistant. Our results are in concurrence with the findings of other studies. High resistance was seen for ampicillin against Gram negative bacilli. Use of ampicillin is in the treatment of UTI in pregnancy is limited because of decreased plasma concentration of up to 50% [6].
Limitation
This study has two limitations: Firstly, pregnant women with bacteriuria were not followed up as the study design was cross-sectional and secondly the subjects were mostly from public hospitals. Further prospective studies should be carried out to understand the impact of ASB on pregnancy outcome and development of pyelonephritis in Saudi Arabia.
Conclusion
The prevalence of ASB in pregnancy in this study was 14.25%, which is relatively higher than reported in other studies. A larger number of women in the third trimester of pregnancy, and multigravida had significant bacteriuria. GBS was the commonest bacterial isolate. This study found that a significant proportion of pregnant women in Saudi Arabia develop ASB. The problem of ASB in pregnancy remains to a considerable extent in Eastern Saudi Arabia, despite the great strides in improvement of the health care delivery system.
AMP: Ampicillin; AUG: Amoxicillin/clavulanic acid; TET: Tetracycline; SXT/TRI: Sulfamethoxazole/trimethoprim; CZN: Cefazolin; FOX: Cefoxitin; GEN: Gentamicin; NF: Nitrofurantoin; PIP/TAZ: Piperacillin/Tazocin; OXA: Oxacillin; VAN: Vancomycin; MOX: Moxifloxacin; CIP: Ciprofloxacin; IMI: Imipenem; CAZ: Ceftazidime
*All MIC values are in μg/ML $MIC values of E.coli are applicable to other Enterobacteriaceae spp.