Glioblastoma Multiforme (GBM) accounts for more than 50% of malignant gliomas. It is known to occur more commonly in elderly population [1]. It has a spectrum of prognostic outcome that is based on age, performance status, extent of surgery, neurological functional class, duration of symptoms and radiotherapy dose as shown in Recursive Partitioning Analysis (RPA) of prognostic factors. According to “The Radiation Therapy Oncology Group- Recursive Partitioning Analysis” (RTOG-RPA), the median survival ranges from 11.1 months for RPA class IV to 4.6 months for RPA class VI [2].
The present standard of treatment is maximal safe resection of the tumour followed by conventional RT to a dose of 60 Gy/30 fractions over 6 weeks. With addition of concomitant and adjuvant Temozolomide, the median overall survival has been shown to improve median Overall Survival (OS) by 2.5 months. However, the lack of survival benefit probably due to increased haematological toxicities in patients with poor performance status has prevented it from being widely accepted world-wide in such patients [3].
Management of this dreadful disease in patients who are elderly with poor mental status is frustrating for the patients, their caregivers and the treating physicians. Reducing the hospital visit of these patients without compromising the outcome would be desirable and would allow better utilization of strained healthcare resources in developing countries. Abbreviated RT course where large dose per day is used so as to complete the treatment in a span of 2-4 weeks has been tried in few studies [4-9].
There is a dearth of data on QOL issues in patients undergoing treatment for GBM. Roa W et al., had used KPS and Functional Assessment of Cancer Therapy-Brain (FACT-Br) questionnaire to assess QOL in their study [4]. There was no significant difference in KPS in the two comparative groups. Severe FACT-Br data was missing in the study. Phillips C et al., had designed a brief neurological function questionnaire to study QOL in their patients subjected to standard dose vs. abbreviated course of RT [5]. Assessment was done at baseline, 2 weeks and 6 weeks of RT and at each clinic review thereafter. However, no formal QOL comparison was reported, due to missing data. QOL issue is even more relevant when survival is limited and other issues of patient’s life are more important. It is imperative that QOL issues are considered integral to the management of this malignancy.
The aim of the study was to determine whether hypofractionated RT is an appropriate treatment option in poor prognosis GBM when compared to standard fractionated RT in terms of survival and quality of life.
Materials and Methods
The study was done between November 2003 and August 2005. The patients were randomly assigned using computer-generated random numbers to receive standard radiotherapy dose of 60 Gy/ 30 fractions over 42 days or 35 Gy/7 fractions/3 fractions per week over 15 days. Sample size of 40 was chosen with 20 patients in each arm [Table/Fig-1].
CONSORT 2010 Flow Diagram.

Patients with the histological proof of Glioblastoma attending the radiotherapy services at Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow were screened for eligibility. Patients who were 50 years and above of any Karnofsky Performance Status (KPS); less than 50 years of age with KPS of 80 or less were included in the study. Patients had to give their informed consent for their participation in the study. Ethical committee clearance was taken to conduct this study.
Radiotherapy simulation was done using 3 clamp thermoplastic casts with three reference marks. RT planning CECT and MRI planning in treatment position were done and the images were transferred to ISIS treatment planning system. The Gross Tumour Volume (GTV) delineation was done on T1W contrast sequence. The GTV was expanded by 3 cm in 3D to define the Planning Target Volume (PTV). Dose prescription was according to International Commission on Radiation Units and Measurements (ICRU) Report 50. Treatment was done on Tele-cobalt unit or Linear accelerator with 6.0 MV photon. RT was started within 3-6 weeks of surgery.
Surveillance and Follow-Up
The patients receiving standard treatment were clinically evaluated once a week while those on short course RT were seen twice a week while on radiotherapy. Subsequently, patients were followed up monthly for the first three months and then every two months. During treatment, a note was made of the steroid and anticonvulsant requirement. On completion of RT, patients were tapered off steroid by reducing 25% of the steroid dose every week but returning to the previous doses in the event of neurological deterioration. Baseline MRI brain was done at three months after RT was over. Subsequently MRI was done on clinical deterioration.
QOL assessment was done by noting down the pre-RT and post-RT KPS, NPS and CPS scores [10]. The difference between the scores at these two times was calculated.
Statistical Analysis
Intention-to-treat analysis method was used. The primary end points were OS and PFS. The secondary end point was QOL as measured by change in score of NPS, CPS and KPS. Differences in proportions in patient disease and treatment characteristics were tested by the chi-square test, while differences in mean were tested by the t-test. OS and PFS were estimated by the Kaplan Meier method and the differences between the curves were tested by the log-rank test. All the endpoints were measured from the date of registration and patients dying of any cause or lost to follow-up were considered as events for both the end points of PFS and OS.
Results
The data was analysed in October 2005. The demographic characteristics are listed in [Table/Fig-2]. The profile in the two arms was comparable except that patients in the control arm were significantly younger than the study arm. The interventions and the compliance of the patients are shown in [Table/Fig-3]. One patient decided to go back home after he was taken in the study; the other deteriorated after the first fraction of RT while the third one succumbed to myocardial infarction during the course of RT.
Result of QOL assessment is shown in [Table/Fig-4]. While the mean KPS scores improved in both the arms by an average of nine points, no differential effect was seen between the two arms. The CPS and the NPS scores worsened minimally, and again, no differential effect was seen for the two arms.
Demographic characteristics.
| Characteristics | Study arm, n=20 (%) | Control arm, n=20 (%) | p-value |
|---|
| Age (years) |
| Mean, SD | 54.4, 9.1 | 46.0, 11.8 | 0.01 |
| Gender | | | |
| Male | 14 (70%) | 15 (75) | 0.72 |
| Female | 6 (30%) | 5 (25) |
| Symptom duration (months) |
| Mean, SD | 9.5, 24.1 | 6.2, 7.6 | 0.56 |
| Seizure duration (months) |
| Mean, SD | 5.5, 4.5 | 4.3, 4.1 | 0.66 |
| Pre-operative KPS |
| 30-50 | 13 (65) | 15 (75) | 0.86 |
| 60-80 | 3 (15) | 3 (15) |
| Unknown | 4 (20) | 2 (10) |
| Pre-radiotherapy KPS |
| 30-50 | 9 (45) | 9 (45) | 0.45 |
| 60-80 | 6 (30) | 8 (40) |
| 90-100 | 5 (15) | 3 (15) |
| Mental status |
| Normal | 9 (45) | 14 (70) | 0.15 |
| Confused | 10 (50) | 6 (30) |
| Unknown | 1 (5) | |
| Pre RT NPS |
| 0-1 | 6 (30) | 6 (30) | 0.78 |
| 2-4 | 13 (65) | 14 (70) |
| Unknown | 1 (5) | |
| Pre RT CPS |
| 0-1 | 6 (30) | 5 (25) | 0.62 |
| 2-4 | 13 (65) | 15 (75) |
| Unknown | 1 (5) | |
SD: Standard deviation; KPS: Karnofsky performance status scale; NPS: Neurological performance scale; CPS: Clinical performance scale; RT: Radiotherapy
Interventions and compliance.
| Interventions/compliance | Study arm, n=20 (%) | Control arm, n=20 (%) | p-value |
|---|
| Surgery |
| Biopsy/Decompression | 13 (65) | 15 (75) | 0.81 |
| Subtotal/ Total | 7 (35) | 5 (25) |
| Surgery–RT interval (days) |
| Mean, SD | 30.4, 13.9 | 31.2, 9.7 | 0.83 |
| Total RT dose (Gy) delivered | 5Gy : 1 (5)15Gy: 1 (5)35Gy: 17 (85)No RT : 1 (5) | 55Gy : 1 (5)60Gy : 19 (95) | |
| Overall treat. time (days) |
| Mean, SD | 13.9, 4.5<14 days : 2 (10)14-16 days : 16 (80)>16 days : 1 (5)No RT : 1 (5) | 45.3, 5.140-44 days: 12 (60)>44 days: 8 (40) | |
RT: Radiotherapy; SD: Standard deviation
Change in KPS, CPS and NPS following treatment.
| Variable | Study arm, n=20 (%) | Control arm, n=20 (%) | p-value |
|---|
| Post-radiotherapy KPS |
| 40-50 | 6 (30) | 6 (30) | 0.37 |
| 60-80 | 4 (20) | 7 (35) |
| 90-100 | 7 (35) | 7 (35) |
| Unknown | 3 (15) | |
| Change in KPS (post RT compared to pre RT) |
| Mean, SD | 9.4, 16.9 | 8.5, 19.0 | 0.89 |
| Post RT NPS |
| 0-1 | 9 (45) | 7 (35) | 0.31 |
| 2-3 | 6 (30) | 12 (60) |
| Unknown | 5 (25) | 1 (5) |
| Change in NPS (post RT compared to pre RT) |
| Mean, SD | -0.6, 1.2 | -0.2, 1.1 | 0.28 |
| Post RT CPS |
| 0-1 | 6 (30) | 9 (45) | 0.30 |
| 2-4 | 9 (45) | 10 (50) |
| Unknown | 5 (25) | 1 (5) |
| Change in CPS (post RT compared to pre RT) |
| Mean, SD | -0.3, 0.9 | -0.2, 1.3 | 0.89 |
KPS: Karnofsky performance status scale; RT: Radiotherapy, SD: Standard deviation; NPS: Neurological performance scale; CPS: Clinical performance scale
Status of patients at last follow-up is shown in [Table/Fig-5]. The median PFS for the study and control arms was 5.5 months vs. 3.8 months (p=0.34) while the median OS was 7.2 months vs. 10.4 months (p=0.09). The survival curves are shown in [Table/Fig-6,7].
Status of patients at the time of analysis.
| Interventions/compliance | Study arm, n=20 (%) | Control arm, n=20 (%) | p-value |
|---|
| Disease progression |
| NED | 6 (30) | 5 (25) | 0.35 |
| Progressed/dead | 14 (70) | 13 (65) |
| LFU, disease status | | 2 (10) |
| Status at last follow-up |
| Alive | 9 (45) | 8 (40) | 0.55 |
| Dead | 9 (45) | 8 (40) |
| LFU after progression | 2 (10) | 2 (10) |
| LFU status | | 2 (10) |
NED: No evidence of disease; LFU: Lost to follow-up
Progression free survival.
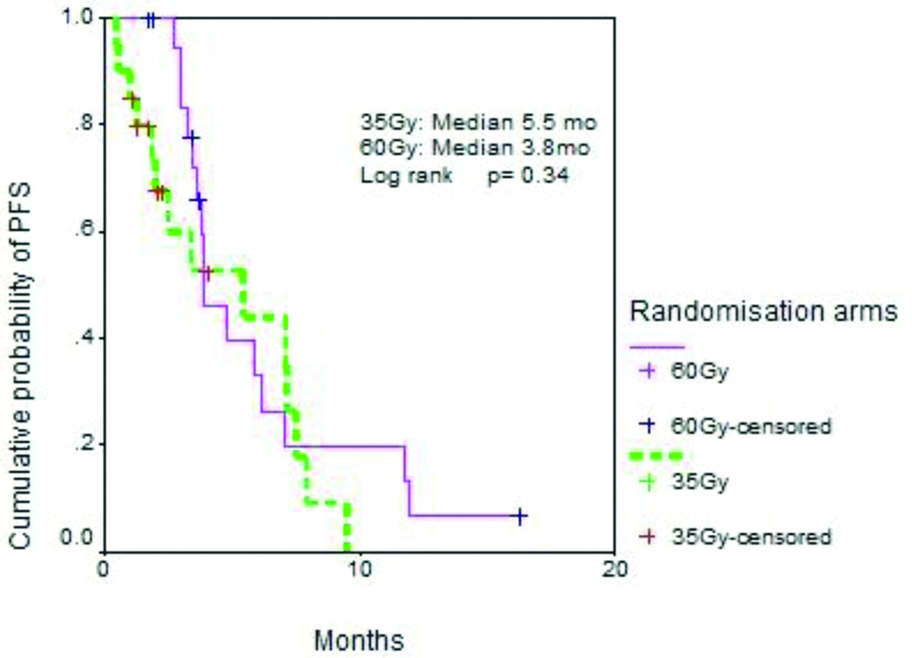
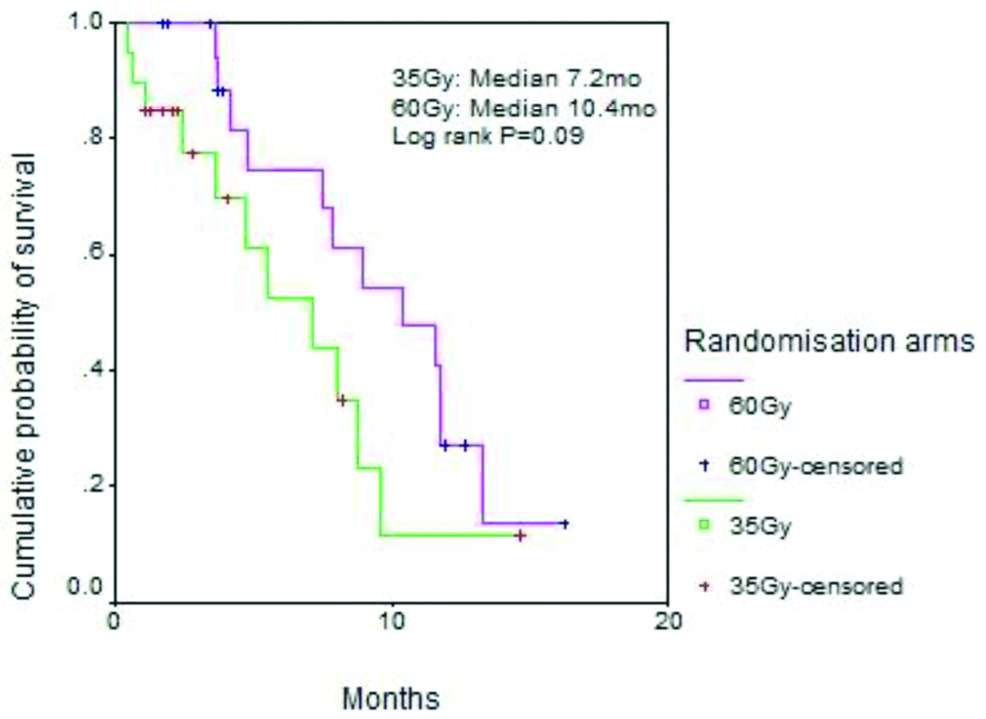
An exploratory analysis was performed to determine which factors amongst the patient, disease and treatment variables, likely influenced probability of survival in the univariate fashion. No factor emerged to be of any significance. A multivariate analysis was therefore not performed.
Discussion
The survival probability given by Curran WJ et al., was based on pre-treatment characteristics of patients i.e., age, mental status, KPS and treatment i.e., extent of surgery and dose of RT. The survival probability of patients of GBM belonging to class IV, V and VI of RTOG-RPA is 11.1, 8.9 and 4.6 months respectively [2]. Authors chose to include patients who were 50 years and above or those with KPS of 70 as the overall short survival time in these groups of patients justifies the use of short course RT protocol.
A perusal of literature on target volume used to treat high-grade gliomas shows an evolving trend in treating lesser volumes with the passage of time and improvements in imaging technology and radiation delivery technology [11].
Patients of GBM with poor prognosis should get best supportive care or should be treated with RT was answered by a phase 3 trial conducted by Keime-Guibert F et al., [12]. A total of 88 patients of GBM, aged 70 years of age and above were randomised into RT arm vs. Best Supportive Care (BSC) arm. RT was tolerated well and had a definite advantage over BSC. Median OS in the RT arm (n=40; 50Gy/28 fractions/38 days) was 28 weeks. Patients (n=44) who received BSC had significantly inferior survival of 17 weeks.
The standard RT protocol for patients of GBM is 60 Gy in 30 fractions over 6 weeks. However, since the prognosis of these patients is heterogeneous, RT dose and schedule should be individualised. Short course hypofractionated RT has an edge over standard six weeks RT for patients of GBM with poor prognostic factors due to the following four reasons. First, the patients and their caregivers will need to spend as less time as possible attending the hospital for treatment. Second, the reduced number of RT sessions confers a cost advantage over standard therapy. Third, it reduces the waiting period over the RT machines and hence allows potentially better utilisation of strained health care resources. Finally, hypofractionated RT exploits the radio-resistance nature of GBM causing increased cell kill and takes care of accelerated tumour cell repopulation by shortening the overall treatment time.
Phillips C et al., prospectively compared standard RT (n=32; 60Gy/30 fractions/6 weeks) with short course RT (n=30; 35Gy/ 10 fractions/2 weeks) in malignant gliomas with poor prognosis. The median survival was 10.3 months in the standard arm and 8.7 months in the experimental arm (p=0.37). Acute toxicities were mild and similar in both the arms. QOL comparison could not be made due to incomplete assessment [5].
Roa W et al., had conducted a prospective trial where histological examination confirmed that new cases of GBM were randomised into control group where standard RT (n= 51; 60Gy/30 fractions/6 weeks) vs. study group i.e., short course RT (n=49; 40Gy/15 fractions/3 weeks). Patients, 60 years and above, with KPS of 50 or more were eligible for the study. The median OS for the two groups did not differ significantly (5.1 months for standard arm and 5.6 months for study arm; p=0.57) [4].
Split course RT with conventional dose per fraction was prospectively compared with hypofractionated RT by Glinski in a phase 3 trial [6]. Of the total 108 patients of malignant gliomas, 44 were of GBM. Patients in the standard arm received 50 Gy in 25 fractions over 5 weeks to the whole brain followed by 10 Gy 5 fractions over 1 week to the tumour bed. Patients in the experimental arm received two courses of 20 Gy in 5 fractions in 1 week at 1 month gap to the whole brain followed by 10 Gy/ 5 fractions over 1 week to the tumour bed. On subset analysis, the 2-year actuarial survival rate was 23% and 10% in experimental and standard arm respectively in patients of GBM and the difference was of statistical significance. However, the median age of these patients was 45 years though the KPS was less than 60 in about 41% of patients. QOL related data was not reported in this trial.
A systemic evidence-based analysis using 23 published articles related to use of radiotherapy in elderly patients with GBM was done by Zarnett OJ et al., [7]. The authors concluded that elderly patients with GBM who are not suitable for combined radiotherapy and Temozolomide should receive either single-agent Temozolomide or hypofractionated radiotherapy alone [7].
A retrospective study using the National Cancer Data Base with GBM patients aged 65 or older was done by Bingham B et al., to independently compare the survival and utilisation rates of different monotherapies. A total of 9556 patients were analysed. The authors had concluded that hypofractionated RT was better than the best supportive care alone and was as good as conventionally fractionated RT alone [8].
Arvold ND et al., had conducted a retrospective analysis on 135 elderly patients of GBM. These patients were treated with conventional RT or hypofractionated RT with or without Temozolomide. There was no difference in survival outcome for patients who had received Temozolomide with conventional RT vs. hypofractionated RT [9].
Stupp R et al., in a landmark randomised controlled trial established the benefit of addition of concomitant and adjuvant Temozolomide to standard RT in patients of GBM. Patients 18-70 years of age and with WHO performance status of 2 or less were considered for the trial. Radiotherapy with Temozolomide was associated with a significant improvement in median OS in nearly all subgroups except those with WHO performance status of 2 [3]. Mirimanoff RO et al., in a RPA of the above trial proved that addition of Temozolomide to RT significantly improved survival for RPA class III and IV but was of only marginal benefit for class V [13]. Therefore, RT with Temozolomide can be advocated for RPA class III and IV but definitely not for class VI and probably not for class V. Moreover, the high cost of the drug prevents its wide spread use in developing countries like India. Therefore, authors chose not to include chemotherapy as a part of treatment for the patients.
QOL issues must be factored in for these poor prognosis patients when judging relative merits of short schedules vs. standard schedules, although this seems to be a difficult enterprise given the cognitive impairment of these patients. Authors used KPS, CPS and NPS as surrogate measure of quality of life. A common problem with other quality of life scales is the inability of patients to complete them, as they are mentally obtunds consequent to their disease. Therefore, authors did not use “Quality of Life Questionnaire” (QLQ) to evaluate QOL in the present study.
Limitation
The poor sample size was the most significant lacunae of the study. Failure to use “Quality of life questionnaire” was perhaps the second limitation of this trial.
Conclusion
Hypofractionated short course RT is equivalent to standard 6 week RT in terms of survival outcome and QOL for poor prognosis patients of GBM. Phase 3 comparative study should be done to see whether addition of Temozolomide to hypofractionated RT improves survival and QOL in these sets of patients.
SD: Standard deviation; KPS: Karnofsky performance status scale; NPS: Neurological performance scale; CPS: Clinical performance scale; RT: Radiotherapy
RT: Radiotherapy; SD: Standard deviation
KPS: Karnofsky performance status scale; RT: Radiotherapy, SD: Standard deviation; NPS: Neurological performance scale; CPS: Clinical performance scale
NED: No evidence of disease; LFU: Lost to follow-up