Metabolic perturbations in diabetes result in detrimental changes in the connective tissues (glycosylation of proteins; micro-vascular abnormalities with damage to blood vessels and nerves; and collagen accumulation in skin and periarticular structures) lead to reduced flexibility [5]. In addition, due to increased systemic inflammatory cytokines, increased muscle protein catabolism, sarcopenia, reduction in maximal aerobic capacity, reduced muscle strength per unit mass in older adults with type 2 diabetes causes reduced skeletal muscle function [6-9]. These patho-physiological changes in muscles due to diabetic process can reduce physiological muscle function in terms of muscle recruitment.
Therefore, it was speculated that altered electrical muscle activity may explain shoulder dysfunction in diabetes, which is not yet explored. Scapulothoracic and glenohumeral muscle activity was studied in people with shoulder impingement and decreased activity of the scapular muscles throughout the arm movements is demonstrated [10,11]. However, to the author’s best knowledge, no studies have delineated differences of muscular activity patterns in patients with diabetes with or without shoulder dysfunction compared to healthy population.
An understanding of the degree to which muscle activity patterns are altered would be relevant to our knowledge of aetiology of pain and stiffness in shoulder in diabetes and may support future directions in clinical settings for prevention and rehabilitation.
Therefore, the purpose of this study was to compare surface muscle activity (EMG) of shoulder muscles and shoulder joint function in patients with diabetes with and without shoulder dysfunction and healthy age matched individuals.
Materials and Methods
This observational cross-sectional study was conducted over two years from March 2015 to May 2017 at Physiotherapy Department, Sancheti Hospital, Pune, Maharashtra, India.
Sample size was estimated using mean value of primary variable sEMG RMS values of shoulder muscles (pectoralis major) during MVC presented by Sandhu JS et al., in a study carried on shoulder muscle activation during push up variations on stable and labile surfaces [12].
To calculate Δ the standardised difference, sometimes called the effect size. In the case of two Means, μ1 and μ2, with a common standard deviation’s’, the standardised difference (s)
Δ=μ1-μ2/s Alternatively, it can be written as:
D or Δ=where; δ is the clinically important difference
In present example, Δ=85.88-51.16/57.34 s=85.88+51.16/2
=34.72 /57.34
=0.60
Using the values from the table for a significance level of 5%, z(1-α/2)=1.96, and a power of 90%, z(1-β)=1.2816,
The number of participants required in each group, m, is given by:
m=2×{z(1-α/2)+z (1-β)}2/Δ2
=2×2.8×2.8/0.6×0.6
=15.68/0.36=43.55 per group
Sample size was estimated to be 43.55 per group (total 3 groups) using sEMG data during MVIC of shoulder muscles as a variable. Therefore, higher sample size (total of 135 samples-45 per group) was used in the present study.
Following approval from institutional ethical committee {Reference No.MGMIHS/RS/2012/49}, the observational, case control study was conducted in compliance with declaration of Helsinki in 1995 (as revised in Edinburgh 2000) [13].
Forty five patients with type 2 diabetes with shoulder dysfunction; 45 patients with type 2 diabetes without shoulder dysfunction and 45 age matched healthy control participants of both genders within age group of 40-65 years were recruited by purposive sampling following signed informed consent at Physiotherapy Department, Sancheti Hospital, Pune. All patients were diagnosed with type 2 diabetes mellitus for more than 5 years. In the present study, shoulder dysfunction was operationally defined as shoulder pain and functional difficulties, with less than 50% limitation in active range of motion (<50%) of elevation and rotation for atleast one-month duration (freezing or frozen stage of frozen shoulder).
Participants in control group were recruited among hospital staff members and people accompanying patients to the hospital. Volunteers were interviewed for including into shoulder dysfunction group, with presence of pain in shoulder or reduced shoulder function during daily work, especially in the overhead position. Patients with history of shoulder surgery, fracture, dislocation and micro trauma to shoulder, impingement syndromes, neurological disorders or chronic cervico-brachial pain symptoms, upper extremity abnormalities or deformities, shoulder pain of cervical origin were excluded.
Demographic information of participants, including name, age, gender, occupation, BMI, duration of diabetes, any history of systemic diseases, medications: oral hypoglycaemic or insulin injection, latest blood sugar level etc., was recorded. Confirmation of diagnosis of type 2 diabetes was based on medical records and previous lab report which consists of criteria of a report of diagnosis as diabetes with the onset after age 25 years; current use of oral/subcutaneous injections hypoglycaemic medications or fasting plasma glucose concentration ≥7.0 mmol/L.
Duration of diabetes was recorded based on recall from the time of diagnosis of diabetes, e.g., for participants with recently diagnosed diabetes, the duration of diabetes was recorded as 0 [14].
Muscle activation of 6 shoulder muscles, namely: clavicular fibers of Pectoralis Major (PM); Biceps (BB); Supraspinatus (SS), Infraspinatus (IS), Upper Trapezius (UT) and Middle Deltoid (MD) was recorded with surface EMG during Maximum Voluntary Isometric Contraction (MVIC) and various functional tasks on dominant or symptomatic side using a dual-channel EMG system (Viking On Nicolet EDX System, 2 channel EMG with Viking Quest electrodiagnostic software.v 20.1.11). Two bipolar surface electrodes were placed at the mid substance of the muscle belly i.e., in between origin and insertion and parallel to the corresponding muscle fibers. A ground electrode was placed over the seventh cervical spinous process. Correct electrode placement was confirmed by EMG activity observed on monitor during a Manual Muscle Test (MMT) for adequate signal processing.
All the participants performed a series of MVIC test for each muscle for normalisation of EMG signals. The tests described by Kelly BT et al., were used for maximal activation of each muscle [15]. Participant applied maximum force in the manual muscle testing position and held it for 5 sec while recording MVICs. All participants were allowed a rest period of 20 seconds in between sEMG recording of MVIC of each muscle. Physiological activity in motor unit during contraction was reflected by Root Mean Square (RMS) value which was used to quantify the electric signal. Myo-electrical signals were recorded in form of MVIC RMS value in μV. The mean of 3 trials of MVICs were computed for selected shoulder muscles for each participant and each muscle as MVIC value.
The same-day test-retest Intra Class Correlation (ICC) for measurement of RMS of pectoralis major and middle deltoid muscle during MVIC from 10 subjects was confirmed by the tester by repeating the measurement on 2 occasions in a day. Intra-class correlation coefficients for recorded EMG values during MVIC ranged from 0.98 to 0.99; indicating high reliability.
Additionally, EMG data were recorded from all 6 muscles during 4 functional tasks, i.e., forward flexion to 900 with 0.5 kg and 2 kg of weight; abduction of shoulder joint to 900 with 0.5 kg and 2 kg of weight. For each task, 3 consecutive trials were recorded and a mean RMS value was obtained. Mean RMS value was used to calculate a percentage of maximal activity for each muscle for normalisation as expressed in the equation below.
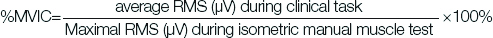
The amount of shoulder muscle activity (RMS values) of these shoulder muscles during four tasks was recorded and converted into percentage of MVIC data recorded for comparison among the three groups. Although %MVIC recorded with sEMG cannot be correlated directly with amount of force produced by muscle; it is still a widely used measure for generating the reference level of maximum muscle activation for normalisation of shoulder muscles EMG activity [16]. This allowed the evaluation of the muscle activity during the task under investigation in comparison to its maximal recruitment. Mean of the average EMG activity (RMS value) during various tasks was normalised to the percentage of MVIC for each muscle and used for further analysis.
Shoulder Pain and Disability Index (SPADI) a shoulder specific self-reported questionnaire was administered by investigator which measured pain, functional activity limitations and disability in patients with shoulder impairments. The filled in questionnaires were collected and filed. The SPADI consists of two dimensions-pain and functional activities associated with shoulder. The pain dimension consists of five questions regarding the severity of an individual’s pain. Functional tasks requiring upper extremities were assessed with eight questions which measured the amount of difficulty a person has during activities of daily living.
Scoring: Subscales were scored in three parts. First, the item scores within the subscales were summed. Second, this sum was divided by the summed distances possible across all the items of the subscale to which the person responded subscales. Third, this ratio is multiplied by 100 to obtain percentage. Higher scores on the subscales indicate greater pain and disability; to obtain SPADI score, pain and disability subscale scores averaged [17].
SPADI score was studied as an indirect measure to indicate shoulder muscle function during activities of daily living.
Statistical Analysis
The Statistical Package for Social Sciences (SPSS) release 20.0 for Windows was used for data analysis. Analysis of Variance (ANOVA) with linear contrast was performed to determine differences and linear trend in mean EMG activity of each muscle during MVIC and tasks and SPADI scores among three groups. Post-hoc analysis for multiple pair wise comparisons (Tukey HSD) was applied during specific comparisons among three groups. Level of significance was set at 0.05.
Results
Demographic data is presented in [Table/Fig-1,2]. Participants of three groups were marginally distributed on age, BMI and duration of diabetes (yr) of cases and controls.
Demographic characteristics of participants among the groups.
| Variables | Healthy Controls mean±SD | Patients with diabetesmean±SD |
|---|
| Group 1 (n=45) | Group 2 (n=45) | Group 3 (n=45) |
|---|
| Gender:Female-n(%)/male-n(%) | 29(64.4%)/16(35.5%) | 27(60%)/18(40%) | 23(51%)/22(49%) |
| Age (yr) | 52.62±9.83 | 53.07±7.60 | 55.29±8.27 |
| BMI (kg/m2) | 24.22±2.54 | 24.19±2.06 | 24.17±4.26 |
| Duration of diabetes (yr) | --- | 7.56±2.64 | 7.87±2.96 |
| Medication (Oral/Insulin) | Oral | Oral | Oral |
| History of hypertension/Thyroid disease | 10/5 | 15/8 | 16/6 |
Distribution of participants based on occupation.
| Occupation | Group 1 | % | Group 2 | % | Group 3 | % |
|---|
| Employee | 16 | 35.55 | 13 | 28.88 | 12 | 26.66 |
| Business | 11 | 24.44 | 5 | 11.11 | 8 | 17.77 |
| Farmer | 3 | 6.66 | 4 | 8.88 | 1 | 2.22 |
| House wife | 11 | 24.44 | 16 | 35.55 | 14 | 31.11 |
| Retired | 3 | 6.66 | 3 | 6.66 | 4 | 8.88 |
| Daily laborer | 1 | 2.22 | 2 | 4.44 | 2 | 4.44 |
| Jobless | 0 | 0 | 2 | 4.44 | 4 | 8.88 |
| 45 | | 45 | | 45 | |
Participants included in all the groups were with or without any other systemic illness e.g., hypertension, that would not affect outcome measures while comparison. Participants with diagnosed diabetes with higher glucose sugar level (fasting and post-meal) or HBA1C level controlled with or without medications were included.
On comparison of mean RMS values of muscle activity during MVIC, significant difference was observed between three groups except infra-spinatus and upper trapezius [Table/Fig-3].
Comparison of RMS of shoulder muscles during MVIC and SPADI score between the three groups.
| RMS (μV) of muscles during MVIC | Group 1 (n=45) Mean±SD | Group 2 (n=45) Mean±SD | Group 3 (n=45) Mean±SD | ANOVA p-value | Linear contrast p-value |
|---|
| Pectoralis major | 136.97±32.17 | 111.48±36.37 | 79.37±21.30 | <0.0001* | <0.0001* |
| Supraspinatus | 133.15±25.70 | 126.15±31.20 | 99.31±32.94 | <0.0001* | <0.0001* |
| Infraspinatus | 114.97±43.08 | 112.31±44.81 | 105.4±41.70 | 0.55 | 0.29 |
| Upper trapezius | 106.13±37.07 | 101.08±48.16 | 96.20±47.96 | 0.57 | 0.29 |
| Biceps Brachii | 97.78±31.53 | 82.97±26.8 | 74.311±27.67 | 0.001* | <0.0001* |
| Middle deltoid | 129.75±2.10 | 117.33±28.47 | 96.48±26.92 | <0.0001* | <0.0001* |
| SPADI Score (%) | 0.20±0.55 | 1.16±2.72 | 59.15±28.55 | <0.0001* | |
*Significance level was set at p<0.05
ANOVA test was used to compare among the groups
Functional status (SAPDI scores) of shoulder joint in patients with diabetes with shoulder dysfunction was found to be significantly different compared to healthy controls. However, no difference was noted in function of shoulder between people with asymptomatic and healthy controls.
On post-hoc analysis using Tukey HSD test, significant difference was revealed in mean RMS values [Table/Fig-4] between group 1 and group 3 and group 2 and 3 in Pectoralis Major (PM), Supraspinatus (SS), Biceps Brachii (BB), Middle Deltoid (MD) muscles except BB in group 2 and 3 during MVIC. On comparing the mean RMS values between groups 1,2 and 3 of infraspinatus and upper trapezius muscles during MVIC was found to be statistically non-significant.
Multiple comparison of mean RMS values during MVIC between group 1, 2 and 3.
| RMS (μV) of muscles during MVIC | Groups | Mean Difference | Std. Error | p-value |
|---|
| Pectoralis major | Group 1 vs Group 2 | 25.48 | 6.45 | <0.0001* |
| Group 1 vs Group 3 | 57.60 | 6.45 | <0.0001* |
| Group 2 vs Group 3 | 32.11 | 6.45 | <0.0001* |
| Supraspinatus | Group 1 vs Group 2 | 7 | 8.01 | 0.65 |
| Group 1 vs Group 3 | 33.84 | 8.01 | <0.0001* |
| Group 2 vs Group 3 | 26.84 | 8.01 | 0.003* |
| Infraspinatus | Group 1 vs Group 2 | 2.66 | 9.11 | 0.95 |
| Group 1 vs Group 3 | 9.57 | 9.11 | 0.54 |
| Group 2 vs Group 3 | 6.91 | 9.11 | 0.72 |
| Upper trapezius | Group 1 vs Group 2 | 5.044 | 9.42 | 0.85 |
| Group 1 vs Group 3 | 9.93 | 9.42 | 0.54 |
| Group 2 vs Group 3 | 4.88 | 9.42 | 0.86 |
| Biceps Brachii | Group 1 vs Group 2 | 14.80 | 6.06 | 0.042* |
| Group 1 vs Group 3 | 23.41 | 6.06 | <0.0001* |
| Group 2 vs Group 3 | 8.66 | 6.06 | 0.328 |
| Middle deltoid | Group 1 vs Group 2 | 12.42 | 6.16 | 0.113 |
| Group 1 vs Group 3 | 33.26 | 6.16 | <0.0001* |
| Group 2 vs Group 3 | 20.84 | 6.16 | 0.003* |
*Significance level was set at p<0.05
ANOVA followed by Post-Hoc analysis–Tukey HSD test was used to compare among the groups
Above results showed a significant reduction in EMG muscle activity of four shoulder muscles during maximal efforts in participants with diabetes with shoulder dysfunction compared to healthy controls. Also significant reduction in muscle activity was observed in patients with diabetes without shoulder dysfunction compared to healthy normal.
However, there was no difference observed in percentage MVIC EMG activity of shoulder muscles during various functional tasks among patients with diabetes with and without shoulder dysfunction compared to healthy shoulder (p>0.05).
Discussion
Present study revealed a linear decline in EMG muscle activity of pectoralis major, supraspinatus, biceps and middle deltoid muscles except infraspinatus and upper trapezius during MVIC from healthy controls to patients with diabetes without shoulder dysfunction to diabetic people with shoulder dysfunction. Further, shoulder muscle activity during MVIC in terms of mean RMS values in patients with diabetes with shoulder dysfunction was significantly lower than healthy matched controls except in infraspinatus and upper trapezius muscle. However, shoulder muscle activity during MVIC in people with diabetes without shoulder dysfunction and healthy controls was different only in pectoralis major and biceps muscle. Additionally, patients with diabetes along with shoulder dysfunction reported functional impairment compared to healthy controls; which was assessed using SPADI.
Patients with diabetes without shoulder dysfunction (healthy shoulder) demonstrated marginally lower average RMS values (shoulder muscle EMG activity) during MVIC compared to healthy participants but the difference was not statistically significant. EMG activity of pectoralis major, supraspinatus, biceps, middle deltoid, infraspinatus and upper trapezius muscle activity during MVIC (maximal amount of recruitment of motor units) was reduced by 18.6%, 5.2%, 15.14%, 9.5%, 3.31% and 4.75% respectively compared to healthy shoulder muscles. Minimal reduction in electrical activity of individual motor units during MVIC (i.e., reduced contractibility) of selected shoulder muscles in asymptomatic normal functioning shoulder is probably because of onset of patho-physiological changes and abnormal skeletal muscle capillary recruitment due to micro-vascular complications in shoulder muscles due to prolonged duration of diagnosed type 2 diabetes (~8 years) [18].
Secondly, EMG activity of pectoralis major, supraspinatus, biceps and middle deltoid activity during MVIC (maximal amount of recruitment of motor units) was reduced by 41.3%, 24.7%, 24% and 25.66% respectively in shoulder dysfunction with diabetes compared to healthy controls. Whereas, minimal decrease of 8.3% and 9.5% in infraspinatus and upper trapezius muscle activity in the symptomatic shoulder with diabetes compared to normal was noted. Reduced EMG muscle activity can be explained by presence of persistent metabolic perturbations in shoulder muscles along with functional limitations like shoulder pain and stiffness in symptomatic shoulder with diabetes. Persistent hyperglycaemia due to diabetes over a minimum of 5 years results in skeletal muscle protein glycation process in which oxidation of sugar produces Advanced Glycation End products (AGE) [19,20]. Elevated AGE levels are known to impair myofibrillar function in type II fibers of skeletal muscle resulting in myosin fiber atrophy and loss of contractibility in addition to impaired micro-circulation in joint tissues in patients with diabetes [14,20-26]. Specific type II fiber atrophy results in reduction in maximal muscle force production can explain the reduction of shoulder muscle EMG activity during MVIC in patients with diabetes [22,23].
It was speculated that, in addition to these metabolic changes in muscles resulting in reduction in muscle activity; the decrease in number of functional motor unit’s activation during maximal muscle contraction due to pain and decreased physical activity revealed by decreased SPADI scores [24]. Reduced motor neuron input due to shoulder dysfunction resulted in almost 25-40% reduction in recruitment of selected shoulder muscles in people with diabetes which is consistent with earlier findings [27].
Pectoralis muscle demonstrated maximal reduction in electrical muscle recruitment ranging from 18-41% whereas; infraspinatus and upper trapezius muscles were least affected. Pectoralis major along with most of the shoulder muscles are reported to consist of 50 to 65% of fast glycolytic and fast oxidative glycolytic muscle fibers (i.e., type II). Higher proportion of glycolytic fibers in pectoralis major can explain reduced maximal muscle recruitment in patients with diabetes. However, infraspinatus and upper trapezius muscle were type I (slow twitch) dominant and found to be least affected [28].
Reduced recruitment of shoulder muscles during MVIC was reflected in marginal decrease in electrical muscle activity (mean% MVIC RMS values) of shoulder muscles in patients with diabetes compared to healthy people during functional task performance. However, it is speculated that these differences were statistically non-significant because of almost similar and systematic recruitment of shoulder muscle patterns or motor strategies with large variations in electrical activity with respect to mean (standard deviations) in all participants owing to their habitual differences. In addition, patients with diabetes with shoulder dysfunction managed to complete the given functional task with pain and difficulty, during the investigation may lead to marginal differences in recruitment patterns. Secondly, isolated maximum recruitment of muscle was demanded during MVIC, whereas functional tasks may not demand maximum recruitment; because of the contribution from other adjacent muscles in varying proportions in patients with or without shoulder dysfunction compared to healthy people. Reduced shoulder muscle recruitment also reflected in decrease in self-reported shoulder function measured with SPADI in people with diabetes with shoulder dysfunction.
Precise EMG findings have increased our understanding of shoulder muscle recruitment pattern among people with diabetes. Recent study in 2018 has demonstrated the importance of lower trapezius strengthening exercises in addition to traditional protocol in patients with frozen shoulder [29]. Similarly, findings from this study can be used to design effective prophylactic muscle strengthening programs targeting pectoralis major, biceps, middle deltoid and supraspinatus muscle strengthening to delay shoulder dysfunction in people with diabetes.
Limitation
One of the limitations was that the participants were matched based on the duration of diabetes, which was done by considering the duration of diabetes on recall basis and calculated from the point of diagnosis. Secondly, for EMG analysis, MVIC method was used for normalisation. The differences in muscle length and the amount of force production may increase slight variations during shoulder muscle contractions during the normalisation and functional tasks.
Conclusion
Shoulder muscles demonstrate linear decline in electrical muscle activity from healthy people to patients with diabetes without shoulder dysfunction to with shoulder dysfunction with maximum affection of pectoralis major muscle. Hence, it is recommended to commence appropriate prophylactic shoulder muscle strengthening exercise program from the onset of diabetes to maximise shoulder function among people with diabetes.
Funding: This research was sponsored by MGM Institute of Health Sciences and did not receive any specific grant from external funding agencies in the public, commercial, or not-for-profit stores.
Authors Contribution
Author 1: Has made substantial contributions to the literature search, acquisition of data, analysis and interpretation of data, drafting and editing the manuscript.
Author 2: Has been involved in the concept, design, and definition of intellectual content, statistical analysis and revising it critically for important intellectual content, literature review, and analysis of the manuscript.
*Significance level was set at p<0.05
ANOVA test was used to compare among the groups
*Significance level was set at p<0.05
ANOVA followed by Post-Hoc analysis–Tukey HSD test was used to compare among the groups