Diffuse Tuberculous Cerebritis in Immunocompetent Hosts-An Uncommon Entity
Animesh Das1
1 Neurology Resident, Department of Neurology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Animesh Das, Neurology Resident, Department of Neurology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow-226014, Uttar Pradesh, India.
E-mail: animeshdas05@gmail.com
Central Nervous System (CNS) tuberculosis is a common disease in developing countries with varied presentation, the most common being tuberculous meningitis. Meningitis, in many cases leads to infection of the underlying cortex resulting in cerebritis, encephalitis or abscess formation. Tuberculous cerebritis has either been described as a focal entity in normal subjects or as a diffuse entity in immunocompromised hosts. We present here two cases of diffuse tuberculous cerebritis in immunocompetent patients. One had presented with left focal seizure with paraparesis while the other presented with non-localising features of raised intracranial pressure. Tubercular Polymerase Chain Reaction (PCR) was positive in Cerebrospinal Fluid (CSF) in the first patient while CSF picture was suggestive of tubercular meningitis in the second, making him a possible case of tuberculosis. Both the patients improved significantly on anti-tubercular drugs.
Arachnoiditis, Central nervous system infection, Paraparesis, Tuberculosis
Case Report
Case-1
A 20-year-old student presented to the outpatient department with two episodes of left sided focal seizure initially followed by progressive diffficulty in walking. He also complained of low backache for two months. There was no history of associated headache, vomitting, fever, altered sensorium or bladder and bowel involvement. There was no history of any drug abuse or high risk behaviour. On neurological examination, he was conscious, oriented with no cranial nerve deficits and neck rigidity. Motor examination revealed asymmetrical lower limb weakness (right>left) with hypotonia and absent reflexes. The sensory system examination was normal. There was no clinical evidence of upper limb or cerebellar involvement.
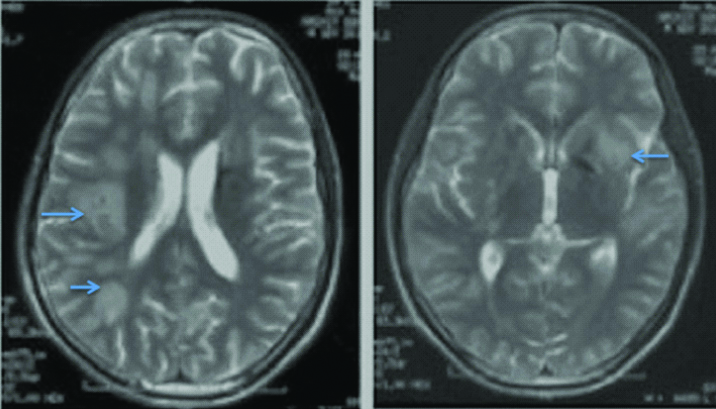
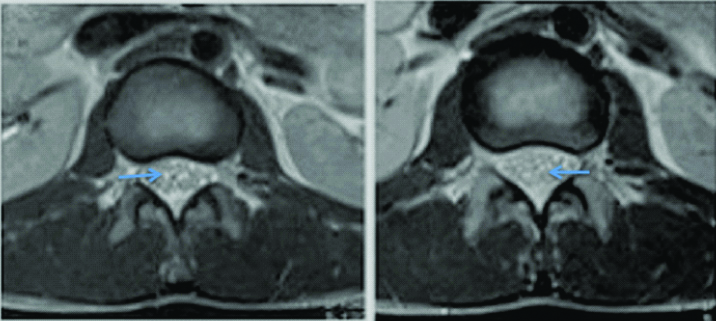
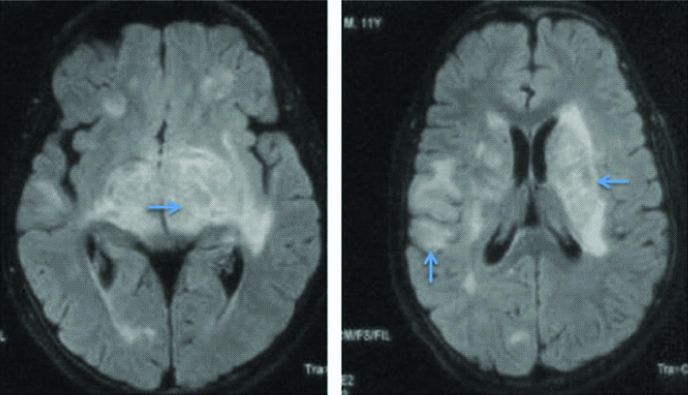
Magnetic Resonance Imaging (MRI) brain revealed bilateral asymmetrical T2 hyperintensities in basal ganglia with punctate hypointensities in between which were gadolinium enhancing. Contrast enhancement was also seen in meninges in gyriform pattern. Cerebrospinal Fluid (CSF) analysis revealed increased opening pressure (28 cm H2O along with raised protein (86 mg/dL), low sugar (58 mg/dL against random blood sugar of 96 mg/dL) and lymphocytic pleocytosis (total cell count of 184 with 70% lymphocytes). CSF examination of Acid Fast Bacilli (AFB) was negative. Cytological examination was negative for malignant cells. Possibility of tuberculous meningitis with tubercular arachnoiditis was kept and repeat MRI brain and lumbosacral spine was done. MRI brain showed focal T2 and flair hyperintensities in bilateral basal ganglia region, corona radiata along with punctate contrast enhancement in gyriform pattern in high parietal area and peripontine cisterns. These findings were suggestive of diffuse cerebral inflammation likely tuberculous cerebritis [Table/Fig-1,2 and 3]. MRI of lumbosacral spine was suggestive of arachnoiditis [Table/Fig-4]. Subsequently, CSF PCR for tuberculous bacilli was found to be positive. Complete blood count and routine biochemistry results were within normal limits. Serology for Human Immunodeficiency Virus (HIV) was negative. Absolute CD4 count was 1100 cells/cu.mm. Radiograph of the chest and ultrasonography of abdomen were normal. CSF culture after four weeks came positive for Mycobacterium tuberculosis sensitive to rifampicin and isoniazid.
T2 weighted images of first patient showing multiple hyper-intensities in bilateral subcortical and periventricular white matter with involvement of basal ganglia.
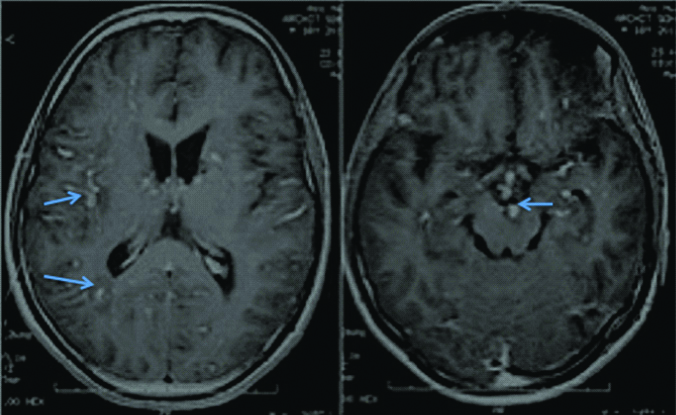
T2 Flair image of first patient showing hyper-intensities in subcortical and periventricular white matter with basal ganglia involvement.

T1 contrast images of first patient showing multiple discrete areas of contrast enhancement in leptomeninges, midbrain, subcortical and periventricular white matter.
Axial T2 images at lumbo sacral level of first patient showing clumping of caudaequina roots, a feature seen in arachnoiditis.
Anti-Tubercular Therapy (ATT) along with steroids (intravenous dexamethasone 0.4,0.3,0.2,0.1 mg/kg/day for four consecutive weeks) was started at admission. Oral levetiracetam (500 mg BD) was given as antiepileptic. At four months follow-up, he was able to walk with support. There were no further episodes of seizure.
Case-2
An 11-year-old boy presented with fever, headache and vomiting for 20 days. There were no localising signs on examination. Fundus revealed no evidence of papilloedema. Neck stiffness and Kernig’s sign was positive.
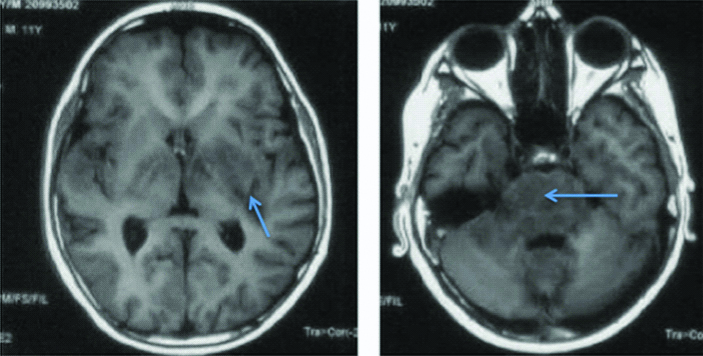
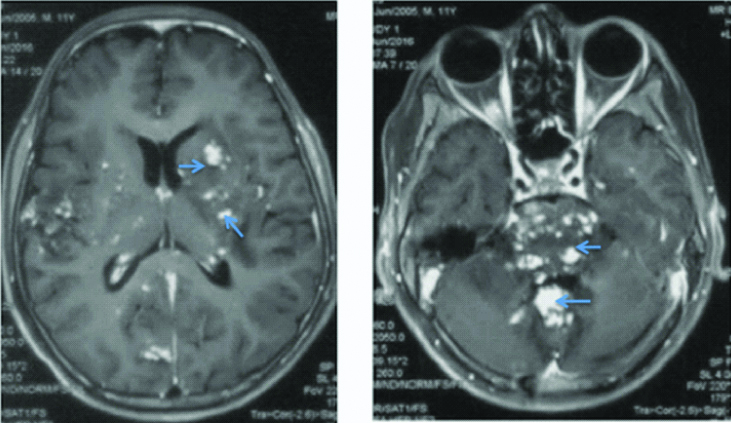
In this patient also, CSF opening pressure was high (26 cm H2O). CSF examination was suggestive of raised protein (434 mg/dL), low glucose (44 mg/dL against blood sugar of 92 mg/dL) and lymphocytic cellularity (total count of 206 with 90% lymphoctes). ZN staining for AFB in CSF was inconclusive. CSF TB PCR and blood CMV PCR were negative. Cryptococcal antigen in CSF was negative by cryptococcal antigen latex agglutination test. CSF for malignant cells was also negative. HIV serology was negative. Absolute CD4 count was 950 cells/cu.mm. Routine haematology and chemistry was normal. MRI brain showed extensive hypointensities in the brainstem, cerebellar peduncles with focal periventricular and subcortical white matter involvement on T1 weighted images [Table/Fig-5]. The corresponding lesions were hyperintense on flair images [Table/Fig-6]. T1 contrast images showed extensive leptomeningeal contrast enhancement with punctate nodular enchancement of basal ganglia, hypothalamus, brain stem and cerebellum, and basal ganglia [Table/Fig-7]. There was no evidence of true diffusion restriction on Diffusion Weighted Imaging (DWI) and corresponding Apparent Diffusion Coefficient (ADC) images. In this patient also, radiograph of the chest and ultrasound abdomen did not show any lymphadenopathy.
T1 weighted images of second patient showing multiple hypo-densities in bilateral basal ganglia, pons, middle cerebellar peduncles and cerebellum.
Flair images of second patient showing multiple hyper-intensities in midbrain, hypothalamus, subcortical and periventricular white matter.
T1 contrast images of second patient showing multiple punctate areas of contrast enhancement in bilateral basal ganglia, subcortical white matter and surrounding grey matter, pons and cerebellum suggestive of cerebritis.
The child was diagnosed as a case of tuberculous cerebritis along with meningitis based on the MRI findings and CSF picture. Other confounding CNS infections like toxoplasma, CMV and HIV were ruled out with relevant serological tests. The patient was started on first line Antitubercular Therapy (ATT) and steroids (IV dexamethasone followed by oral prednisolone) with significant improvement in symptoms within two months. At one year, he had total neurological recovery without any residual deficit.
Discussion
Various clinico-radiological presentations of CNS tuberculosis have been previously reported. Tubercular meningitis, tuberculomas, hydrocephalus, basal exudates, tuberculous abscess are more commonly described than tuberculous cerebritis which may be due to the fact that very few patients present at this stage and it is common to miss the subtle imaging findings of this stage. Cerebritis may be the preceding event in the development of tuberculomas or tubercular abscesses but such cases usually get missed either due to late presentation on the part of the patient or poor reporting on the part of the treating clinician. Focal tuberculous cerebritis in an immunocompetent host was first described by Jinkins while diffuse tuberculous cerebritis was described by Abrishamkar in a HIV patient [1,2]. Diffuse tuberculous cerebritis in an immunocompetent host has never been described to the best of our knowledge.
Jinkins while describing focal tuberculous cerebritis, proposed that the loss of cerebral autoregulation, increase in vascularity and breakdown of blood brain barrier is responsible for the typical imaging pattern of focal cerebritis in which contrast enhanced CT scan shows gyral enhancement and angiograpy shows palisading blush [1]. Cerebritis precede the development of abscess formation [3]. Pathologically, there are patchy areas of microgranulomata around minor foci of tuberculous bacilli in cerebritis phase. MRI brain may show ill-defined hypointense or isointense gyri on T1, hyperintense foci on T2 with diffuse patchy post-contrast enhancement [4]. There may be areas of restricted diffusion [5]. This is different from imaging pattern of miliary CNS tuberculosis, another form of diffuse parenchymal involvement by tubercular bacilli. Multiple tiny T2 hyperintensities less than 2 mm infiltrating the brain surface around the corticomedullary junction which are invisible on plain T1 and enhance on gadolinium is the characteristic MRI finding of this miliary form [6].
In any case of cerebritis, the further course depends on variety of factors like the number of bacilli, immunity status of the host and antimicrobial agent used. In case of an abscess being formed, the subacute symptoms of CNS tuberculosis like low grade fever, headache change into more sinister symptoms of focal neurological deficits, altered sensorium, cranial nerve palsies. The imaging findings also vary according to the stages of non-caseating granulomas to caseating granulomas and abscesses. Non-caseating granulomas are iso/hypo intense on T1, hyperintense on T2 with homogenous contrast enhancement [7,8]. Caseating solid granulomas are both T1 and T2 iso/hypointense which may enhance on contrast administration [9]. But frequently, these lesions have surrounding oedema which is hypointense on T1 and hyperintense on T2, the wall of the lesion shows out as hypointense rim. Caseating granulomas with liquefactive necrosis becomes centrally hyperintense on T2 in contrast to solid granulomas [8].
Our first case had features of basal ganglia infarcts with meningeal inflammation before the development of tuberculous cerebritis in subsequent MRI. The second case had proper tuberculous cerebritis on imaging right at presentation to our centre.
Conclusion
These cases highlight one of the rare presentations of a common disease. MRI brain with diffuse punctate enhancement of cerebral cortex and no evidence of ring enhancing lesion or abscess is quite characteristic of cerebritis and in appropriate circumstances like CSF positivity for tubercular bacilli or response to ATT favours tubercular etiology.
[1]. Jinkins JR, Focal tuberculous cerebritisAm J Neuroradiol 1988 9(1):121-24. [Google Scholar]
[2]. Abrishamkar S, Tuberculous cerebritis and tuberculoma in a patient with AIDS: Literature review and case reportJournal of Research in Medical Sciences 2006 11(4):273-77. [Google Scholar]
[3]. Kim SH, Choi SS, Kang MJ, Evolution of caseating granuloma from tuberculous cerebritis in the corpus callosumHong Kong J Radiol 2015 18:46-50.10.12809/hkjr1414270 [Google Scholar] [CrossRef]
[4]. Falcone S, Post M, Encephalitis, cerebritis, and brain abscess: Pathophysiology and imaging findingsNeuroimaging Clin N Am 2000 10:333-53. [Google Scholar]
[5]. Höllinger P, Zürcher R, Schroth G, Mattle HP, Diffusion magnetic resonance imaging findings in cerebritis and brain abscesses in a patient with septic encephalopathyJ Neurol 2000 247:232-34.10.1007/s00415005057310787125 [Google Scholar] [CrossRef] [PubMed]
[6]. SaneiTaheri M, Karimi MA, Haghighatkhah H, Pourghorban R, Samadian M, DelavarKasmaei H, Central nervous system tuberculosis: An imaging-focused review of a reemerging diseaseRadiol Res Pract 2015 2015:20280610.1155/2015/20280625653877 [Google Scholar] [CrossRef] [PubMed]
[7]. Dastur DK, Mangheni DK, Udani PM, Pathology and pathogenetic mechanisms in neurotuberculosisRadiol Clin North Am 1995 33:733-52. [Google Scholar]
[8]. Gupta RK, Pandey R, Khan EM, Mittal P, Gujral RB, Chhabra DK, Intracranial tuberculomas: MRI signal intensity correlation with histopathology and localised proton spectroscopyMagn Reson Imaging 1993 11:443-49.10.1016/0730-725X(93)90079-S [Google Scholar] [CrossRef]
[9]. Poptani H, Gupta RK, Roy R, Pandey R, Jain VK, Chhabra DK, Characterization of intracranial mass lesions with in vivo MR proton spectroscopyAm J Neuroradiol 1995 16:1593-91. [Google Scholar]