Introduction
Vitamin D deficiency leads to defective mineralisation and in Chronic Kidney Disease (CKD) can contribute to renal osteodystrophy.
Aim
To compare the serum level of vitamin D in children with CKD as compared to those with normal condition.
Materials and Methods
This cross-sectional survey was performed on 68 consecutive children with CKD and 73 sex and age-matched healthy children who were referred to Mofid hospital in Tehran, Iran in 2016. Serum level of vitamin D was measured by immunoassay kits and was then categorised as ≤15 ng/dL (for vitamin D deficiency), 16-30 ng/dL (for vitamin D insufficiency) and >30 ng/dL (for normal condition). Also, the CKD stage was determined by measuring estimated Glomerular Filtration Rate (GFR). Quantitative variables were compared with the t-test, Mann-Whitney test, ANOVA test, or Kruskal-Wallis H test. Qualitative variables were also compared with chi-square test or Fisher’s-exact test.
Results
Comparing serum level of vitamin D between CKD and control group showed a significant difference (14.3±9.8 ng/mL versus 18.3±10.9 ng/mL, p<0.001). To investigate relationship between level of vitamin D and stages of CKD, authors determined CKD stages. In CKD group, 5.9% were categorised as Stage I, 20.6% as Stage II, 19.1% as Stage III, 19.1% as Stage IV, and 35.3% as Stage V. In CKD and healthy children, vitamin D deficiency was revealed in 69.1% and 42.5%, and vitamin D insufficiency in 20.6% and 38.4% respectively (p=0.006). Interestingly, the serum level of vitamin D had an inverse association with the CKD stage. By increasing disease stage, the prevalence of vitamin D deficiency gradually increased (p=0.005). Authors found a direct association between CKD stage and serum level of parathormone. Also, the mean level of parathormone was higher in those children with vitamin D deficiency (376.6±296.4 ng/L) as compared to those with vitamin D insufficiency (292.1±259.5 ng/L) and those with normal level of vitamin D (61.4±31.9 ng/L) (p=0.001).
Conclusion
Vitamin D deficiency is a common problem among children with CKD compared to control group. In CKD patients vitamin D level is decreased with increasing CKD stage.
Chronic renal insufficiency, Paediatric, Parathormone, Renal osteodystrophy
Introduction
CKD is known as a global public health problem in almost all countries and is fatal, if remained uncontrolled and unmanaged. In the recent two decades, despite significant development in diagnostic and therapeutic approaches, the incidence of CKD has been steadily increasing in both adults and children, especially in less developed countries [1-3]. The global prevalence of childhood CKD has been reported in the range of 18.5-58.3 per million children [4]; however, it may be underreported due to lack of recognition or the absence of proper data registries in third world countries [5].
Unmanaged CKD can be a life-threatening event because of its adverse cardiovascular consequences as well as its shifting to renal failure [6]. Particularly in children, the risk of delayed growth and development may be also expected and thus following early recognition and treatment of CKD is strongly recommended [7]. Unfortunately, in some countries, more than the two-third of affected children with CKD develop End-Stage Renal Disease (ESRD) that leads to a long-term survival of <80.0%, in future stages of their lives [8]. The main causes of death in those children are cardiovascular defects (ischaemic events, arrhythmias and cardiac arrest), pulmonary oedema, and hyperkalemia [9,10]. Moreover, most affected cases with ESRD require long-term dialysis or renal transplantation that may also lead to higher mortality and morbidity [11]. Along with cardiovascular and metabolic consequences of CKD, the potential adverse effect of CKD on mineralisation is very impressive. The children affected by CKD are at increased risk for altered bone formation and strength due to both renal osteodystrophy and also vitamin D deficiency [12]. In this regard, Kidney Disease Improving Global Outcomes (KDIGO) have suggested regular measuring serum 25-hydroxyvitamin D (25[OH]D) levels in children affected by CKD [13]. Vitamin D deficiency in CKD children can suppress RAAS system, modulate immune system and inflammation and may reduce its progression [14]. Hence, the present study aimed to compare the serum level of vitamin D in children with CKD as compared to those with normal condition.
Materials and Methods
This cross-sectional survey was performed on 68 consecutive children with CKD during two years and 73 sex and age-matched healthy children who were referred to Mofid Hospital in Tehran, Iran in 2016. The study was approved by the ethics committee at Shahid Beheshti University of Medical Sciences (IR.SBMU.MSP.REC.1395.32). The sample size was calculated using the variables: P1: 34.9% [15], P2: 58% [16], α: 5%, β: 20%; sample size was calculated to be 68.
These patients were collected during 24 consecutive months (January 2016 to January 2018). The baseline characteristics of children including sex, age, medical history, medications were collected by interviewing the parents. In both groups, anthropometric parameters including height and body weight were assessed by single standard tools. In CKD group, the aetiologies for disease were also collected through assessment of available diagnostic checklists such as laboratory parameters and radiology and ultrasonography reports. Also, those suspected of cardiovascular events due to CKD were assessed by complementary diagnostic tools such as two-dimensional echocardiography. In all subjects, serum level of vitamin D was measured by immunoassay kits and was then categorised as ≤15 ng/dL (for vitamin D deficiency), 16-30 ng/dL (for vitamin D insufficiency) and >30 ng/dL (for normal condition) [17].
Also, the CKD stage was determined by measuring estimated GFR as Stage I (GFR >90 with normal kidney function), Stage II (GFR 60 to 89 with mildly reduced kidney function), Stage III (GFR 30 to 59 with moderately reduced kidney function), Stage IV (GFR 15 to 29 with severely reduced kidney function), and Stage V (GFR <15 with very severe, or end-stage kidney failure).
Statistical Analysis
For the statistical analysis, the statistical software SPSS version 16.0 for windows (SPSS Inc., Chicago, IL) was used. Results were presented as mean±Standard Deviation (SD) for quantitative variables and were summarised by absolute frequencies and percentages for categorical variables. Normality of data was analysed using the Kolmogorov-Smirn-off test. Categorical variables were compared using chi-square test or Fisher’s-exact test when >20% of cells with expected count of <5 were observed. Quantitative variables were also compared with t-test, Mann-Whitney test, ANOVA test, or Kruskal-Wallis H test. p-values of 0.05 or less were considered statistically significant.
Results
In CKD and healthy groups, 61.2% and 58.9% were males respectively (p=0.863) with the average age of 6.4±4.5 years and 6.7±3.7 years respectively (p=0.761). The mean height was significantly lower in CKD group than in healthy children (104.9±31.7 cm versus 134.7±9.3 cm, p<0.001). Similarly, the mean weight was also significantly lower in former group (20.3±12.6 kg versus 39.7±12.2 kg, p<0.001).
Comparing serum level of vitamin D between CKD and control group showed a significant difference between them (p<0.001) which is shown in [Table/Fig-1].
Mean serum level of vitamin D in CKD and control group.
| group | N | Mean | Std. Deviation | Std. Error Mean |
|---|
| Vitamin D | case | 68 | 14.2559 ng/mL | 9.84694 ng/mL | 1.19412 |
| control | 131 | 18.3580 ng/mL | 10.89104 ng/mL | 0.95155 |
In CKD and healthy control children, vitamin D deficiency was revealed in 69.1% and 42.5%, vitamin D insufficiency in 20.6% and 38.4%, and normal vitamin D level in 10.3% and 19.1% respectively, with a significant difference (p=0.006).
Patients in CKD group were categorised into five stages according to severity of CKD. Interestingly, the serum level of vitamin D had an inverse association with the CKD stage and severity (p<0.001) in [Table/Fig-2].
Mean serum level of vitamine D according to stage of CKD.
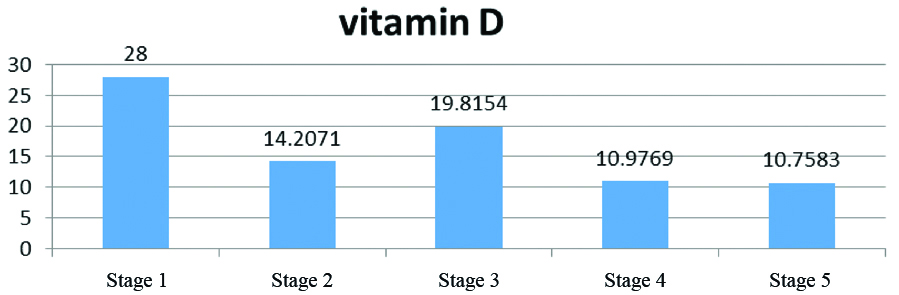
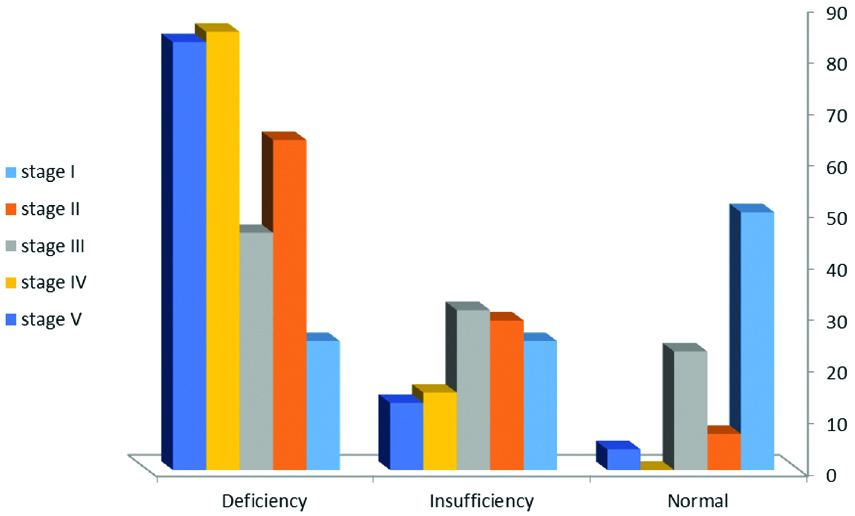
Similarly, as shown in [Table/Fig-3], by increasing disease stage, the prevalence of vitamin D deficiency gradually increased (p=0.005).
The association between vitamin D deficiency and CKD stages.
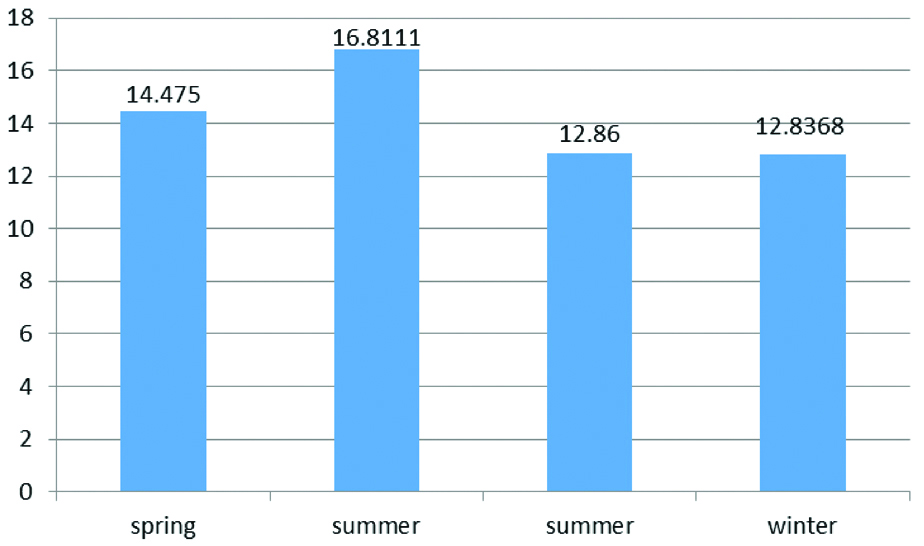
The serum level of vitamin D was independent to the season in which patients were refered (p=0.056) which is depicted in [Table/Fig-4].
Mean serum level of vitamin D of patients in different seasons.
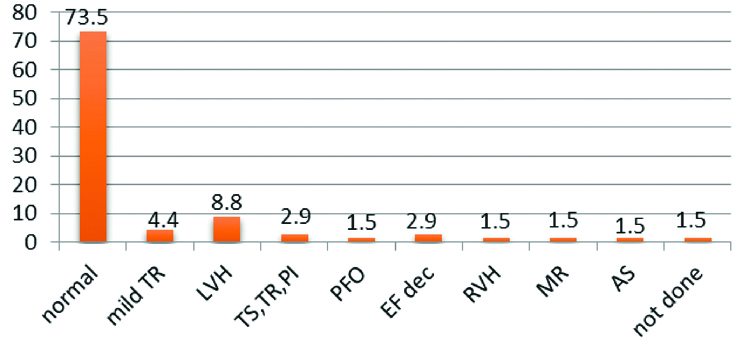
Different aetiologies of CKD are shown in [Table/Fig-5]. The most common of them was neurogenic Cardiovascular consequences of CKD, depicted in [Table/Fig-6] that shows left ventricular hypertrophy as the most common one.
Distribution of different etiologies of CKD.
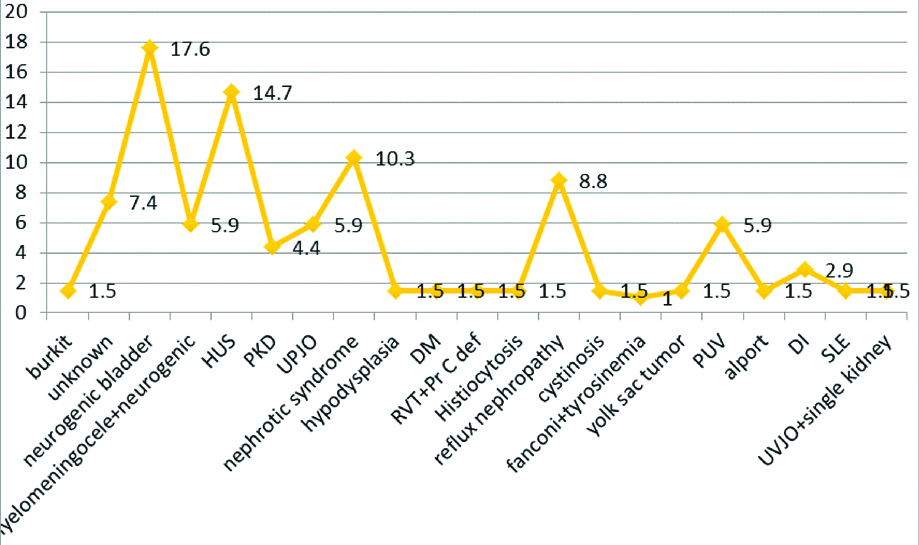
Distribution of different etiologies of CKD.
The highest and the lowest level of vitamin D was found in CKD children who were candidate for renal transplantation and those who underwent haemodialysis [Table/Fig-7], however the difference was insignificant (p=0.266).
The level of vitamin D and its deficiency according to CKD treatment plan.
| No treatment (55.8%) | Peritoneal dialysis (26.5%) | haemodialysis (10.3%) | Renal transplantation (7.4%) | p-value |
|---|
| Serum level of vitamin D (ng/mL) | 16.4±10.5 | 11.1±7.0 | 7.7±4.2 | 18.3±13.3 | |
| Vitamin D status | | | | | 0.266 |
| Normal | 5 (13.2) | 1 (5.6) | 0 (0.0) | 1 (20.0) | |
| Insufficiency | 11 (28.9) | 2 (11.1) | 0 (0.0) | 1 (20.0) | |
| Deficiency | 22 (57.9) | 15 (83.3) | 7 (100) | 3 (60.0) | |
| n | 38 | 18 | 7 | 5 | |
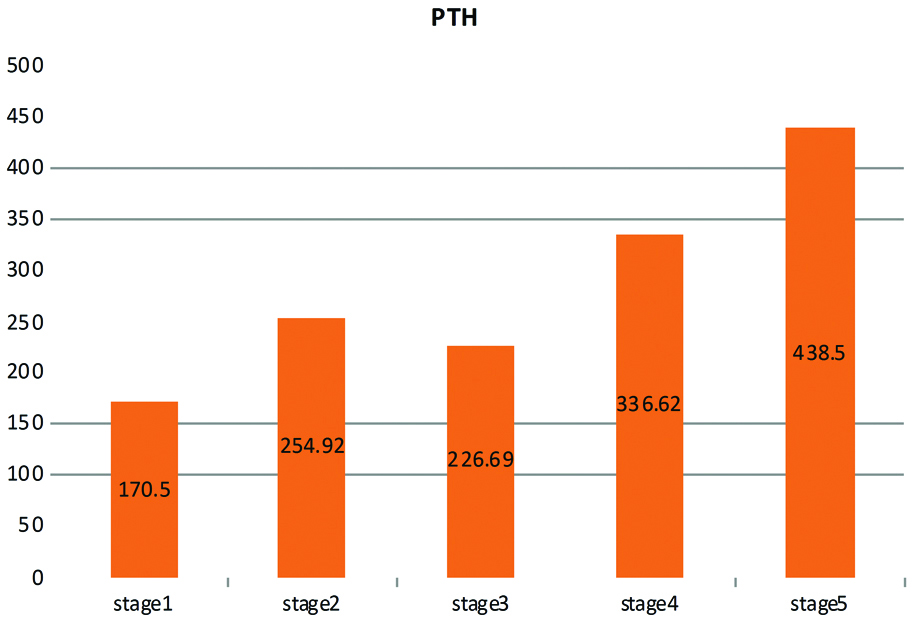
Authors found a direct association between CKD stage and serum level of parathormone as shown in [Table/Fig-8].
Mean serum PTH according to different stages of CKD.
Also, the mean level of parathormone was higher in those children with vitamin D deficiency (376.6±296.4 ng/L) as compared to those with vitamin D insufficiency (292.1±259.5 ng/L) and those with normal level of vitamin D (61.4±31.9 ng/L) (p=0.001).
Discussion
In the present study, increased prevalence of vitamin D deficiency is clearly shown when CKD stage increases. Along with the adverse association between serum vitamin D and stage of CKD, the level of parathormone was also directly associated with the severity of vitamin D insufficiency. Nowadays concern about role of vitamin D in many diseases has increased [18]. As shown by Menon S et al., it was found that 77 % of CKD patients had vitamin D deficiency or Insufficiency [19]. Similar to present observation, Seeherunvong W et al., showed a higher prevalence of vitamin D deficiency and insufficiency in those with more advanced CKD [20]. In the present survey, the overall prevalence of vitamin D deficiency in CKD children was 69.1% that was consider high when compared to previous reports. As shown by Kumar J et al., in India [21], it was revealed a 28 % prevalence of 25O HD deficiency at enrollment. It has been also indicated in other reports [22,23]. The higher prevalence of vitamin D deficiency among the study children might be due to poor nutritional habits, improper lifestyle and sun exposure especially among girls, and inappropriate physical activities though it needs further investigations. The association between the degree of vitamin D deficiency and CKD stages can be explained. First, the paediatric patients with more advanced stage of CKD might be under a restricted diet and physical activity, and vitamin D deficiency is more common [24]. Second, the patients with advanced CKD and overt proteinuria might have greater loss of urinary vitamin D metabolites which can influence inadequate vitamin D status such as vitamin D binding protein [25]. The third explanation is that serum 1, 25(OH)2D functions as a negative endocrine regulator of renin gene expression, and inadequate vitamin D status may affect the development of hypertension and progression of CKD [26].
In the present study, lower vitamin D was associated with higher concentration of parathormone. In fact, a negative association can be expected between serum level of vitamin D and parathormone that was also previously noticed [24]. These data suggest that vitamin D deficiency contributes to the development of hyperparathyroidism in CKD. In fact, it seems that in children with higher stages of CKD, consuming vitamin D supplements can be associated with delay in the onset of hyperparathyroidism [23]. According to some guidelines such as the KDOQI, it is recommended to measure vitamin D levels in stage 2-4 of CKD children [27,28]. Gonzalez EA et al., showed that inadequate vitamin D status was associated with secondary hyperparathyroidism in patients with CKD even without dialysis [29]. In the present study, PTH levels negatively correlated with vitamin D levels. KDIGO guidelines recommend to evaluate 25(OH) D levels and correct abnormalities, if PTH is found more than upper normal limit of assay in CKD stage 3-5 [30]. There has been no consensus for acceptable PTH levels in each stage of CKD. Some authors recommended the cut-off value of 30.27 ng/mL for 25(OH) D to predict a PTH level above 70 pg/mL [31].
In the present study, there was no association between vitamin D deficiency and season of assessment [Table/Fig-4]. This finding is contradictory to some reports but was similar to others. As shown by Stein DR et al, Kumar J et al., and Ali FN et al., there was no difference in 25(OH) D levels between those enrolled during the sunny months and those enrolled during non-sunny months [24,32,33], that was contrary to some other studies indicating more prevalence of vitamin D deficiency during the winter months [34,35]. These paradoxical findings may be associated with the different geographical characteristics in different regions and different climates.
Limitation
Limitation of this study was that authors couldn’t follow-up the vitamin D deficient patients and measure their vitamin D level after treatment with supplemental vitamin D.
Conclusion
In summary, Vitamin D deficiency is a common finding among children with CKD. A lower level of vitamin D is expected in children with higher stages of the disease.
Abbreviation
25(OH) D: 25 Hydroxy Vitamine D; ANOVA: Analysis of variance; CKD: Chronic kidney disease; PTH: Parathormon; UPJO: Ureteropelvic junction obstruction
[1]. ABecherucci F, Roperto RM, Materassi M, Romagnani P, Chronic kidney disease in childrenClin Kidney J 2016 9(4):583-91.10.1093/ckj/sfw04727478602 [Google Scholar] [CrossRef] [PubMed]
[2]. Kari J, Epidemiology of chronic kidney disease in childrenJ Nephropathol 2012 1(3):162-63.10.5812/nephropathol.811324475409 [Google Scholar] [CrossRef] [PubMed]
[3]. El Nahas AM, Bello AK, Chronic kidney disease: the global challengeLancet 2005 365:31-40.10.1016/S0140-6736(05)17789-7 [Google Scholar] [CrossRef]
[4]. Ledermann S, Rees L, Shroff , Long-term outcome of chronic dialysis in children. In: Wardy BA, Schaefer F, Jexander SR, edsPediatric Dialysis 2014 2nd edNew York, NYSpringer:645-660.10.1007/978-1-4614-0721-8_33 [Google Scholar] [CrossRef]
[5]. Ardissino G, Dacco V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, Marra G, Edefonti A, Sereni F, Epidemiology of chronic renal failure in children: data from the ItalKid projectPediatrics 2003 111:e382-e387.10.1542/peds.111.4.e38212671156 [Google Scholar] [CrossRef] [PubMed]
[6]. Mitsnefes MM, Cardiovascular disease in children with chronic kidney diseaseJ Am Soc Nephrol 2012 23(4):578-85.10.1681/ASN.201111111522383696 [Google Scholar] [CrossRef] [PubMed]
[7]. Bao R, Chen CY, A retrospective study on nutritional status and growth and development of 37 children with chronic kidney disease stage 3 to 5Chinese Journal of Pediatrics 2016 54(9):674-78. [Google Scholar]
[8]. Baum M, Overview of chronic kidney disease in childrenCurr Opin Pediatr 2010 22:158-60.10.1097/MOP.0b013e32833695cb20299869 [Google Scholar] [CrossRef] [PubMed]
[9]. Greenbaum LA, Warady BA, Furth SL, Current advances in chronic kidney disease in children:growth, cardiovascular, and neurocognitive risk factorsSemin Nephrol 2009 29:425-34.10.1016/j.semnephrol.2009.03.01719615563 [Google Scholar] [CrossRef] [PubMed]
[10]. Chavers BM, Li S, Collins AJ, Herzog CA, Cardiovascular disease in pediatric chronic dialysis patientsKidney Int 2002 62:648-53.10.1046/j.1523-1755.2002.00472.x12110030 [Google Scholar] [CrossRef] [PubMed]
[11]. Fadrowski JJ, Frankenfield D, Amaral S, Brady T, Gorman GH, Warady B, Children on long-term dialysis in the United States: Findings from the 2005 ESRD clinical performance measures projectAm J Kidney Dis 2007 50:958-66.10.1053/j.ajkd.2007.09.00318037097 [Google Scholar] [CrossRef] [PubMed]
[12]. Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney diseaseKidney Int 2007 71:31-38.10.1038/sj.ki.500200917091124 [Google Scholar] [CrossRef] [PubMed]
[13]. KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD), Kidney IntSupplements 2017 7(1):1-59(1)10.1016/j.kisu.2017.04.00130675420 [Google Scholar] [CrossRef] [PubMed]
[14]. Shroff R, Wan M, Rees L, Can vitamin D slow down the progression of chronic kidney disease?Pediatr Nephrol 2012 27(12):2167-73.10.1007/s00467-011-2071-y22160397 [Google Scholar] [CrossRef] [PubMed]
[15]. Rabbani A, Alavian SM, Motlagh ME, Ashtiani MT, Ardalan G, Salavati A, Vitamin D insufficiency among children and adolescents living in Tehran, IranJ Trop Pediatr 2009 55(3):189-91.10.1093/tropej/fmn07818775944 [Google Scholar] [CrossRef] [PubMed]
[16]. Belostotsky V, Mughal MZ, Berry JL, Webb NJ, Vitamin D deficiency in children with renal diseaseArch Dis Child 2008 93(11):959-62.10.1136/adc.2007.13486618463127 [Google Scholar] [CrossRef] [PubMed]
[17]. Lee JY, So T-Y, Thackray J, A review on vitamin d deficiency treatment in pediatric patientsThe Journal of Pediatric Pharmacology and Therapeutics: JPPT 2013 18(4):277-91.doi:10.5863/1551-6776-18.4.27710.5863/1551-6776-18.4.27724719588 [Google Scholar] [CrossRef] [PubMed]
[18]. Esfandiar N, Alaei F, Fallah Sh, Babaie D, Sedghi N, Vitamin D deficiency and its impact on asthma severity in asthmatic childrenItal J Pediatr 2016 42:10810.1186/s13052-016-0300-527987538 [Google Scholar] [CrossRef] [PubMed]
[19]. Menon S, Valentini RP, Hidalgo G, Peschansky L, Mattoo TK, Vitamin D insufficiency and hyperparathyroidism in children with chronic kidney diseasePediatr Nephrol 2008 23:1831-36.10.1007/s00467-008-0842-x18575896 [Google Scholar] [CrossRef] [PubMed]
[20]. Seeherunvong W, Abitbol CL, Chandar J, Zilleruelo G, Freundlich M, Vitamin D insufficiency and deficiency in children with early chronic kidney diseaseJ Pediatr 2009 154:906-11.10.1016/j.jpeds.2008.12.00619230902 [Google Scholar] [CrossRef] [PubMed]
[21]. Kumar J, McDermott K, Abraham AG, Friedman LA, Johnson VL, Kaskel FJ, Prevalence and correlates of 25-hydroxyvitamin D deficiency in the Chronic Kidney Disease in Children (CKiD) cohortPediatr Nephrol 2016 31(1):121-29.10.1007/s00467-015-3190-726307635 [Google Scholar] [CrossRef] [PubMed]
[22]. Kalkwarf HJ, Denburg MR, Strife CF, Zemel BS, Foerster DL, Wetzsteon RJ, Vitamin D deficiency is common in children and adolescents with chronic kidney diseaseKidney Int 2012 81:690-97.10.1038/ki.2011.43122205356 [Google Scholar] [CrossRef] [PubMed]
[23]. Seeherunvong W, Abitbol CL, Chandar J, Zilleruelo G, Freundlich M, Vitamin D insufficiency and Deficiency in children with early chronic kidney diseaseJ Pediatr 2009 154(6):906-11.e1.10.1016/j.jpeds.2008.12.00619230902 [Google Scholar] [CrossRef] [PubMed]
[24]. Stein DR, Feldman HA, Gordon CM, Vitamin D status in children with chronic kidney diseasePediatr Nephrol 2012 27:1341-50.10.1007/s00467-012-2143-722453735 [Google Scholar] [CrossRef] [PubMed]
[25]. Grymonprez A, Proesmans W, Van Dyck M, Jans I, Goos G, Vitamin D metabolites in childhood nephrotic syndromePediatr Nephrol 1995 9:278-81.10.1007/BF022541837632510 [Google Scholar] [CrossRef] [PubMed]
[26]. Li YC, Vitamin D regulation of the renin-angiotensin systemJ Cell Biochem 2003 88:327-31.10.1002/jcb.1034312520534 [Google Scholar] [CrossRef] [PubMed]
[27]. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summaryAm J Kidney Dis 2009 53(3 Suppl 2):S11-104.10.1053/j.ajkd.2008.11.01719231749 [Google Scholar] [CrossRef] [PubMed]
[28]. Shroff R, Wan M, Gullett A, Ledermann S, Shute R, Knott C, Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: a randomized trialClin J Am Soc Nephrol 2012 7:216-23.10.2215/CJN.0476051122266572 [Google Scholar] [CrossRef] [PubMed]
[29]. Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ, Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational studyAm J Nephrol 2004 24:503-10.10.1159/00008102315452403 [Google Scholar] [CrossRef] [PubMed]
[30]. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD)Kidney Int Suppl 2009 (113):S1-130. [Google Scholar]
[31]. Lee KW, Lee ST, Cho H, Optimal vitamin D levels in children with chronic kidney diseaseJ Nephrol Ther 2015 5:207 [Google Scholar]
[32]. Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML, Prevalence and associations of 25-Hydroxyvitamin D deficiency in US Children: NHANES 2001-2004Pediatrics 2009 124(3):e362-e70.10.1542/peds.2009-005119661054 [Google Scholar] [CrossRef] [PubMed]
[33]. Ali FN, Arguelles LM, Langman CB, Price HE, Vitamin D deficiency in children with chronic kidney disease: uncovering an epidemicPediatrics 2009 123:791-96.10.1542/peds.2008-063419255004 [Google Scholar] [CrossRef] [PubMed]
[34]. Van der Mei IA, Ponsonby AL, Engelsen O, Pasco JA, McGrath JJ, Eyles DW, The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitudeEnvironmental Health Perspectives 2007 115(8):1132-39.10.1289/ehp.993717687438 [Google Scholar] [CrossRef] [PubMed]
[35]. Kull M Jr, Kallikorm R, Tamm A, Lember M, Seasonal variance of 25-(OH) vitamin D in the general population of Estonia, a Northern European countryBMC Public Health 2009 9:2210.1186/1471-2458-9-2219152676 [Google Scholar] [CrossRef] [PubMed]