NPTCL comprise a rare and heterogeneous group of mature T-cell lymphomas that include PTCL-NOS, ALCL-ALK+, ALCL-ALK- and AITL [1].
Large-scale studies have enriched the understanding of these uncommon lymphomas. Two important examples being the Non-Hodgkin Lymphoma Classification Project and the International PTCL Project (the results of which are accepted and quoted in the 2008 World Health Organisation (WHO) classification). They have stated that the PTCLs comprise a wide range of 1.5-20% of all NHLs over many countries. The International PTCL Project, in fact, specifies that PTCLs comprise 5-10% of all NHL in western countries and 15-20% in Asian continent [2-4].
Two previous studies from the present centre showed varying prevalence of 15% (1997-2000) and 9% (2003-04) [5,6]. A need to compile and update the number of recent cases of these rare and neglected group of lymphomas was felt. Incidence not withstanding, other reasons to try and gain insight into these lymphomas came from the fact that Immunohistochemistry (IHC) is often confusing in many cases due to the immunophenotypic aberrations and receptor rearrangement studies are not widely prevalent in developing countries [7]. Therefore, familiarity with the usual as well as the unusual heterogeneous histopathological features would be imperative for diagnosis. This is crucial, given the tendency of these lymphomas to be aggressive, widespread at diagnosis with higher clinical stage and poor treatment response [8].
Thus, to evaluate NPTCL, a cohort study was designed, with the aim of documenting the clinico-radiological, histopathological and follow-up parameters of all NPTCL diagnosed in a single tertiary care centre in Southern India over five and a half year duration.
Materials and Methods
Search through archives had shown a total of 91 cases of NPTCL being diagnosed between January 2008 and June 2013 in the Department of Pathology, JIPMER, Puducherry, India. Eleven cases were excluded from subsequent study due to review of diagnosis (two cases) and technical factors (nine cases). Hence, the final sample size of this descriptive cohort study on NPTCL was 80 patients for whom the histopathological and clinico-radiological details were collected and compiled in a retrospective (48 cases, 2008-2011) and prospective (32 cases, 2012-2013) manner.
Clearance from the Institute Research Ethics Committee was sought and obtained (Letter number: SEC/2011/4/31). Written informed consent was obtained from patients for enrolling them in the study. Relevant clinical details like age, gender, type of lymphadenopathy and groups of lymph nodes involved, B symptoms and other complaints, extranodal organ involvement, radiological findings, skin infiltration, hepatosplenomegaly, bone marrow involvement and peripheral smear details were all collected from the various sources. Clinical Stage was determined as per Cotswold modification of Ann Arbor staging system [9,10]. Wherever data was not available or unsuitable for analysis, it was suitably coded for exclusion. Follow-up data was collected from the records available with the Departments of Medical Oncology or the Medical Records Department. Wherever, phone numbers were available, an effort was made to contact the patient to know their current status.
For cases diagnosed from December 2011, sections of 2 to 3μ thickness were made from all the formalin fixed paraffin embedded blocks and stained with Haematoxylin and Eosin stain (H&E) as per standard procedure [11]. For the older cases, the retrieved H&E slides were screened in every case. Re-staining with fresh sections was done if necessary. Reticulin stain, Periodic Acid Schiff (PAS) stain was done as per protocol in all cases of AITL and as per need in other cases [11]. Reticulin stain would highlight areas of parenchymal fibrosis and PAS stain, by its property of staining the thickened basement membrane, would highlight the increased high endothelial venules in AITL, providing improved contrast for ease of study. Fibrosis in a lymph node supports lymphoma and may be confused at times with necrosis. Performing Reticulin stain would solve the dilemma as only fibrotic areas would get highlighted.
On H&E sections of both the archived and the fresh cases, the histomorphological parameters studied were:
Effacement of node architecture (complete or partial)
Perinodal infiltration (present or absent)
Vascularity (increased or normal)
Predominant size of neoplastic cells (small, intermediate or large)
Predominant reactive cells (eosinophils, macrophages, lymphocytes, plasma cells)
Necrosis (present or absent)
Mitosis (an arbitrary cut off of 10/10 high power fields or more was taken as high mitotic rate)
Fibrosis (present or absent)
Pleomorphism (present/absent)
Histologic pattern (conventional/variant)
An arbitrary cut-off was used based on identifying non-lymphomatous background cells that contribute at least 25% of all the counted cells in three high power fields from two geographically distant foci within the neoplastic lymph node. Such a population was branded as “predominant reactive population”.
Immunohistochemical confirmation of the diagnosis of NPTCL was obtained in all the cases. The lineage determining (B-cell vs. T-cell origin) markers used for all cases were CD2, CD3, CD4, CD5, CD7, CD8 and CD20. Among these, CD20 detects B-cells and the rest are used to detect T-cells. CD20 is the most sensitive marker to detect B-cells and CD3 for T-cells. The diagnosis oriented markers, used as appropriate, were CD30 and ALK-1 (for ALCL), and CD10, CD23 and Bcl-6 (for AITL). In addition, Ki-67 was done in all cases to assess the degree of proliferation of tumour cells. Bone marrow aspirates and trephine biopsies were retrieved from departmental archives wherever available and studied for presence of infiltration. In bone marrow biopsies, pattern of infiltration was also noted. Miscellaneous marrow findings were recorded and suitably coded.
Statistical Analysis
Statistical analyses were performed using SPSS software (Version 16.0, SPSS Inc., Chicago, IL). Chi-square (χ2) test or Fisher-exact test were used to look for association of subtypes of NPTCL with clinical features and the clinico-pathological parameters with Stage IV disease. A p-value less than 0.05 was considered as statistically significant.
Results
During the study period from January 2008 to June 2013, 1282 lymph node biopsies were received in the Department of Pathology of which NPTCL comprised 89 cases. These 89 cases of NPTCL equated to 18% of all Lymphomas, 25% of all NHL, 75% of all T-NHL and 7% of all lymph node biopsies received in the centre.
Ultimately, as described above, 80 cases of NPTCL were included in the present study of which 41 were of the PTCL-NOS group, 23 of the ALK-positive ALCL group, and eight each of the ALK-negative ALCL and AITL groups.
The demographic distribution of various NPTCL are shown in [Table/Fig-1]. AITL and ALK-negative ALCL predominantly involved the elderly and the younger population respectively. Of the 80 cases, only six patients were children (<12 years). A lone girl was a case of PTCL-NOS. The five boys comprised two cases each of PTCL-NOS and ALK-positive ALCL while one was diagnosed as an ALK-negative ALCL.
Demographic details of the nodal peripheral T-cell lymphoma patients.
| Diagnosis(n=number of patients) | Demographic details |
|---|
| Median age (in years) | Range (in years) | Male | Female | M:F ratio |
|---|
| No. of patients | % | No. of patients | % |
|---|
| PTCL-NOS(n=41) | 47 | 2-87 | 30 | 73 | 11 | 27 | 2.7:1 |
| ALCL, ALK+(n=23) | 24 | 5-70 | 18 | 78 | 5 | 22 | 3.6:1 |
| ALCL, ALK-(n=8) | 28 | 5-58 | 4 | 50 | 4 | 50 | 1:1 |
| AITL(n=8) | 57.5 | 45-68 | 6 | 75 | 2 | 25 | 3:1 |
| Total(n=80) | 43.5 | 2-87 | 58 | 72 | 22 | 28 | 2.6:1 |
The clinical profile of patients among various sub-groups are mentioned in [Table/Fig-2]. Most patients presented with node swellings as their chief complaint (76%). B-symptoms were most common with ALK-negative ALCL (75%).
Clinical profile across the four subtypes of the nodal peripheral T-cell lymphoma patients.
| Type of Lymphoma (n=Total number of patients) | Presenting Complaints |
|---|
| Duration of Presenting complaints | % of patients |
|---|
| Median (in months) | Range (in months) | Node swelling | B symptoms | Abd.* | Resp.† |
|---|
| PTCL-NOS(n=41) | 3 | 0.1-15 | 71 | 37 | 17 | 20 |
| ALCL, ALK+(n=23) | 2 | 0.5-6 | 83 | 57 | 30 | 4 |
| ALCL, ALK-(n=8) | 2.5 | 0.7-12 | 88 | 75 | 38 | 0 |
| AITL(n=8) | 2 | 0.25-6 | 75 | 63 | 38 | 13 |
| Total(n=80) | 3.9 | 0.1-15 | 76 | 49 | 13 | 13 |
*Abd.=Abdominal symptoms; †Resp=Respiratory symptoms
Overall, 54 patients (69%) presented with generalised palpable lymphadenopathy [Table/Fig-3]. The two patients without palpable lymphadenopathy, had intra-abdominal nodes sonographically. Half of the ALCL-ALK- patients had only localised lymphadenopathy, the most in any sub-group. Cervical lymph nodes were the commonest group of lymph nodes involved in NPTCL. In descending order of frequency of involvement, the other groups of involved nodes were axillary, intra-abdominal, inguinal, intra-thoracic, supraclavicular, epitrochlear and popliteal lymph nodes.
Profile of organomegaly, lymphadenopathy and groups of lymph nodes involved in patients of nodal peripheral T-cell Lymphoma.
| Diagnosis (n=number of patients) | in % of patients |
|---|
| Peripheral lymphadenopathy | Groups of nodes involved | Organomegaly |
|---|
| a* | l† | g‡ | C§ | A|| | I** | IA†† | IT‡‡ | S§§ | a* | HSM||| |
|---|
| PTCL, NOS (n=41) | 2 | 39 | 59 | 85 | 53 | 50 | 53 | 32 | 3 | 68 | 15 |
| ALCL, ALK+ (n=23) | 4 | 18 | 78 | 86 | 52 | 29 | 60 | 13 | 24 | 48 | 39 |
| ALCL, ALK- (n=8) | 0 | 50 | 50 | 89 | 38 | 13 | 33 | 33 | 13 | 62 | 25 |
| AITL (n=8) | 0 | 0 | 100 | 100 | 100 | 100 | 75 | 25 | 0 | 37 | 50 |
| Total (n=80) | 2 | 30 | 68 | 87 | 56 | 43 | 59 | 25 | 10 | 59 | 26 |
*a=Absent; †I=Localised; ‡g=generalised; §C=Cervical; ||A=Axillary; **I=Inguinal; ††IA=Intra-abdominal; ‡‡IT=Intra-thoracic; §§S=Supraclavicular; |||HSM=Hepatosplenomegaly
Hepatosplenomegaly was seen in 26% of all the patients [Table/Fig-3]. Radiology helped detect splenic infiltration in two patients with no palpable splenomegaly.
Skin infiltration was seen in six patients (8%) comprising three from PTCL-NOS, one from ALK-positive ALCL and two patients of AITL.
Erroneous histopathological diagnosis of Hodgkin lymphoma and empirical therapy for clinically suspected Tuberculosis were unusual settings of patient referral. In addition, six patients were either relapse or recurrence in known cases of NPTCL. Two patients were found to be retropositive on further workup.
Complete peripheral smear findings were available in 65 patients. Of them, three patients (5%) showed atypical cells in peripheral blood. Eosinophilia and Neutrophilia were noted in 32% of the smears each. Cytopenias (isolated, bi- or pancytopenias) was seen in 12% of the smears. Anaemia (72% of the cases) was not obligatory in the disease and was mostly of the normocytic normochromic type. Overall, peripheral smear findings were non-specific and did not appear to offer any significant clue in diagnosis.
To briefly summarise the histomorphological features [Table/Fig-4], two-thirds of the node biopsies of patients displayed perinodal infiltration and diffusely effaced node architecture. Increased vascularity, as expected, was seen maximally in AITL patients. Necrosis, intranodal fibrosis and high mitosis were more often seen in ALCL. Statistical tests showed that PTCL-NOS (Pearson’s χ2 correlation, p-value=0.014) and AITL (Fisher’s exact test, p-value=0.013) were more likely to be associated with Stage IV disease. The p-values for association of parameters such as B-symptoms, necrosis, reactive eosinophils, fibrosis with Stage IV disease were 0.275, 0.660, 0.861 and 0.108 and, therefore, were not significant. In fact, none of the individual histomorphological features correlated statistically to adverse outcome measured with regard to Stage IV or advanced stage (Stage III and Stage IV) disease.
Histopathological findings among the patients of nodal peripheral T-cell lymphoma.
| Diagnosis(n=numberof patients) | Histological Parameters (in % of cases) |
|---|
| PI* | DE† | IV‡ | Fibrosis | Necrosis | Mitosis§ | Variant|| | Predominant reactive population |
|---|
| E** | H†† | L‡‡ | P§§ |
|---|
| PTCL-NOS (n=41) | 70 | 78 | 54 | 22 | 15 | 42 | 19 | 20 | 61 | 17 | 2 |
| ALCL, ALK+ (n=23) | 78 | 91 | 65 | 52 | 26 | 48 | 26 | 44 | 26 | 17 | 4 |
| ALK-ALCL (n=8) | 87 | 87 | 75 | 50 | 38 | 62 | 25 | 62 | 38 | 0 | 0 |
| AITL (n=8) | 75 | 87 | 87 | 25 | 13 | 38 | 0 | 38 | 50 | 0 | 12 |
*PI=Perinodal infiltration; †DE=Diffuse effacement; ‡IV=Increased vascularity; §Mitosis=Classified as high or low on the basis of 10 mitotic figures/10 high power fields; ||Variant=Variant morphological patterns; PTCL-NOS=5 Lennerts; 2 Interfollicular; 1 Follicular; ALCL-ALK+=2 null cell, 1 small cell, 1 lymphohistiocytic, 1 Nodular sclerosis like; ALCL-ALK-=1 small cell; 1 Nodular sclerosis like; **E=Eosinophils; ††H=Histiocytes; ‡‡L=Lymphocyte; §§P=Plasma cells
Bone marrow biopsy or aspiration showed infiltration in 24 of the 64 patients, in which it was done (38%). In 13 patients, infiltration was made out only on trephine biopsy and not on the aspirate smears. A 66% of the patients had an interstitial pattern of infiltration. Of the 33 patients with normocytic normochromic anaemia, 15 (48%) showed bone marrow involvement, significant finding (Pearson’s chi-square correlation, p-value=0.014). Most common marrow finding irrespective of marrow involvement was eosinophilia, either alone or associated with other reactive leukocytic changes (29% of all the marrows). Haemophagocytosis, osteomyelosclerosis and myelonecrosis with fibrosis were uncommon marrow findings.
Follow-up and outcome data are displayed in [Table/Fig-5]. It was seen that among all the groups, at least 70% of the patients who could be staged were in advanced stage of the disease (Stages III & IV). The median follow-up duration for all subgroups was 17 months with a range of 0-72 months in the 40 patients who had followed-up in the present hospital. Data on current status of patients could be obtained in only 30 patients. Of these, 10 patients had died of the disease while two were unwell with the disease. The rest are currently healthy. Nine of the dead patients were having advanced disease at initial presentation (seven with Stage IV and two with Stage III) and four of them had, in fact, received upfront palliative oral chemotherapy or supportive medications. However, when an attempt was made to correlate patients with Stage IV disease alone and adverse clinical outcome (unwell or dead) at last follow-up, the correlation was not statistically significant (p-value=0.288).
Clinical Staging and follow-up status among the patients of nodal peripheral T-cell lymphoma with available data*.
| Diagnosis | Clinical stage (ann arbor) (in % of patients) | Follow-up duration (in months) | Current status of patients (in % of patients) |
|---|
| I | II | III | IV | Median | Range | Well | Unwell | Dead† |
|---|
| PTCL–NOS | 12 | 18 | 33 | 37 | 15.5 | 7-49 | 77 | 8 | 15 |
| ALCL, ALK+ | 0 | 19 | 24 | 57 | 22 | 0-37 | 33 | 0 | 67 |
| ALK-ALCL | 17 | 0 | 17 | 66 | 15 | 6-52 | 50 | 25 | 25 |
| AITL | 0 | 0 | 0 | 100 | 17 | 11-72 | 75 | 0 | 25 |
| Total | 8 | 15 | 26 | 51 | 17 | 0-72 | 60 | 7 | 33 |
*The numerical values for follow-up duration and current status of patients are calculated keeping in mind a total of 30 patients that include 13,9,4 and 4 patients of PTCL-NOS, ALK-positive ALCL, ALK-negative ALCL and AITL respectively for whom the data was available while Clinical Stage is calculated for 41,23,8 and 8 patients of PTCL-NOS, ALK-positive ALCL, ALK-negative ALCL and AITL; † the values reflect the case fatality rate and represent the mortality risk of the disease
Discussion
The prevalence of NPTCL was seen to be higher in the current study (25% of all NHLs) compared to WHO 2008 (7%) as well as Western literature (10-15%) [2,4,12]. In the present centre this represents an increased incidence of at least 10% over the past decade as data from a 1997-2000 study of Naresh KN et al., equates to NPTCL comprising 15% of all NHL back then [5]. Data from other parts of Southern India is variable. In a recently published study from a tertiary care centre in Vellore, a town 150 km from the present centre, the incidence of PTCL overall was found to be 17.4% over the period of 2008-09 while the NPTCL incidence was comparable with the WHO (2008) data of 7% [13]. Yet, a recent study from another Southern state of Kerala had a mild increase in NPTCL that contribute roughly 11% of all NHL [14]. The selective increase in incidence continues to confound the authors. One possible reason could be the fairly homogenous patient population of JIPMER whereas CMC, Vellore receives a more dispersed patient population with a significant chunk being contributed by non-local population [15]. Rest of India generally reports a wide variation in prevalence ranging from near equal to the WHO (2008) value to triple its value (and, therefore, comparable to the present reported value of 25%): North-6% (Dubey AP et al.,) and 6.9% (Gogia A et al.,), West-8.7% (Naresh KK et al.,) and 9.8% (Sahni CS et al.,), East-15.2% (Mondal SK et al) and North East-20% (Devi et al) [16-21].
To purely speculate, tropical nations are known for infectious diseases, which often require the mounting of a T-cell mediated immune response to contain the offending pathogens. With this background, one may hypothesise that the increased proliferation and activation of T-cells necessary for an immune response would be a reason leading to accumulation of grave mitotic errors and lymphomagenesis. Perhaps environmental factors, for example, viral triggers such as Epstein-Barr virus or Human Herpes Virus-6, that are known carcinogens and widely prevalent in tropics, are the cause for this increased incidence. Detailed epidemiological and microbiological studies across different geographic locations could, perhaps, throw more light.
The observed median age of ALK-negative ALCL (28-years) was lower than that described in literature (40-65 years) [2,22]. Though WHO 2008 does state that these lymphomas can occur at any age, this might represent a geographic variation and warrants further study [2,22,23].
In the present study, more than two-thirds of the patients in each subgroup were in advanced stage disease. This was along expected lines [2,23-27].
Data on follow-up in the present centre was difficult to come by. As many as 50 patients had been lost to follow-up after the initial biopsy or even after undergoing few cycles of chemotherapy. Of the rest, one third of the patients had died of the disease. Thus lack of satisfactory data in more than half of the study cohort prevented the authors from attempting survival statistics. In fact, [Table/Fig-5] is based only on 13 and 9 patients of PTCL-NOS and ALK-positive ALCL and four patients each of ALK-negative ALCL and AITL. Strangely, case fatality rate appeared to be highest among the ALK-positive ALCL group of patients. Low numbers of patients in that subgroup may have contributed to the apparent adverse risk of the entity. Also, 9 of the 10 dead patients were in advanced disease i.e., Stage III and Stage IV at presentation. Thus, as per the present study, advanced stage of disease appears to be the only strong influence on mortality. No, individual histopathological parameters appeared to affect mortality significantly.
Clinically, the present authors observed generalised lymphadenopathy in a majority of cases and peculiarly cervical nodes were enlarged in at least 85% of all patients. It would not be too unwieldy to propose the observation as a clue towards an infectious aetiology having an oronasal portal of entry. B symptoms are said to be more frequently seen in ALCL as was seen in 75% of the cases [23]. Radiology was crucial in the assessment of intra-abdominal and intra-thoracic lymph nodes and also helped detect splenic involvement in two patients with no palpable splenomegaly. However, no specific pattern of organ infiltration or visceral nodal involvement was detected in any of the four entities of NPTCL in the present study.
The three patients who had peripheral blood involvement had unique characteristics. Two represented the youngest and oldest patients of PTCL-NOS. The third was a lady with lymphohistiocytic variant of ALK-positive ALCL, a variant commonly described as having association with spill [28]. AITL patients strangely did not show spill contrary to reports [29,30]. Seventy-two percent of the patients were anaemic. Presence of normocytic normochromic anaemia correlated with bone marrow infiltration in the study (χ2 test, p-value=0.040).
Bone marrow infiltration is seen in 14-50% of PTCL-NOS, 10-30% of ALCL and in 54-90% of the AITL cases [23,31-34]. Marrow imprint smears often contained more density of the atypical lymphoid cells than aspirate smears. In only 13 of the 24 cases with biopsy proven marrow infiltration was the marrow aspirate positive for infiltration. This underscores the importance of a thorough screening of imprint smears and waiting till the completion of IHC on a trephine biopsy before ruling out marrow infiltration. Haemophagocytosis in marrow was seen in two patients and both were diagnosed as ALK-positive ALCL. However, literature states that it is more common in PTCL-NOS [2].
The classical microscopic findings of ALCL are hallmark cells (embryo like nucleus), richly vascular node effaced by lymphoma cells that often have abundant clear cytoplasm in the perivascular location in AITL and high grade lymphoma features often with eosinophil and macrophage rich background population in PTCL-NOS [Table/Fig-6,7 and 8]. However, classic descriptions are neither constant nor diagnostic. Histologically, the two morphological differential diagnoses that had to be considered most of the times on review of slides were T-cell/Histiocyte rich B-cell lymphoma (TCRBCL) and Hodgkin lymphoma. Also, there was frequent disagreement over the histological subtype of NPTCL on morphology. A polymorphous population of cells favours NPTCL over TCRBCL whereas Reed-Sternberg cells favour a Hodgkin lymphoma since the background lymphocytes lack pleomorphism unlike in NPTCL. Even then, IHC is the final arbiter. The [Tables/Fig-9,10] represent a simple prototype to identify CD3 positivity and CD20 negativity (except in residual follicles) in cases of NPTCL.
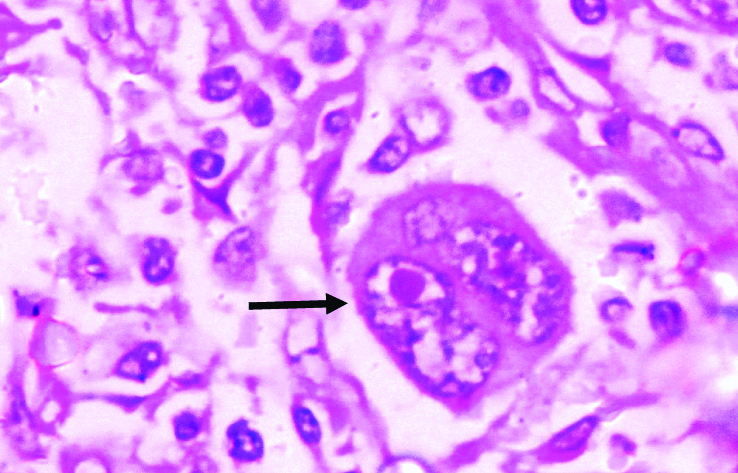
Hallmark cells in a case of anaplastic large T-Cell lymphoma resembling embryos or convoluted kidneys. The cells and the nuclei are large with vesicular chromatin and prominent, mostly single, eosinophilic nucleolus (H&E, 1000X).
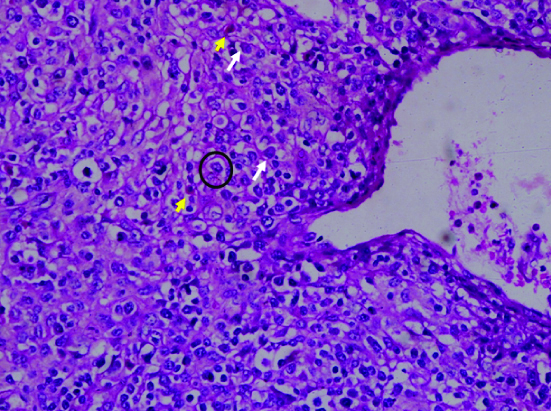
Perivenular neoplastic lymphoid cells with abundant clear cytoplasm (circle with black outline) in a case of angioimmunoblastic T-cell lymphoma. note the scattered eosinophils (yellow arrows) and plasma cells (white arrows) in the background milieu. (H&E, 100X).
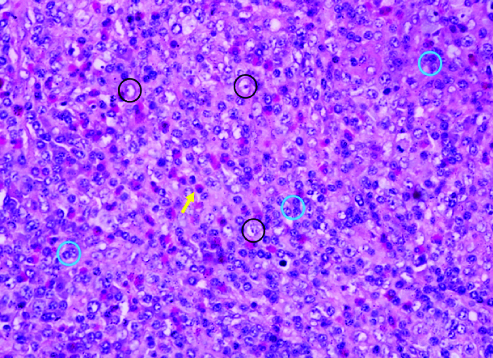
A case of lennert lymphoma with intimate admixture of lymphoma cells (circles with blue outline) and macrophages (circles with black outline) along with a prominent population of eosinophils (yellow arrow) that are a useful diagnostic clue in histomorphology of T-cell lymphomas. (H&E, 100X).
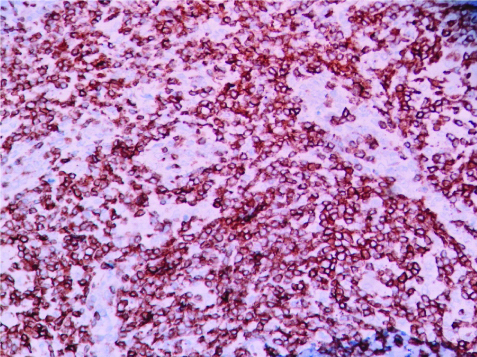
CD3 positivity in a case of PTCL-NOS. (IHC, 100X).
A case of AITL showing a destroyed follicle (evidenced by CD20 positive cells- black arrow) due to infiltration by lymphoma cells (yellow arrow). (IHC,100X).
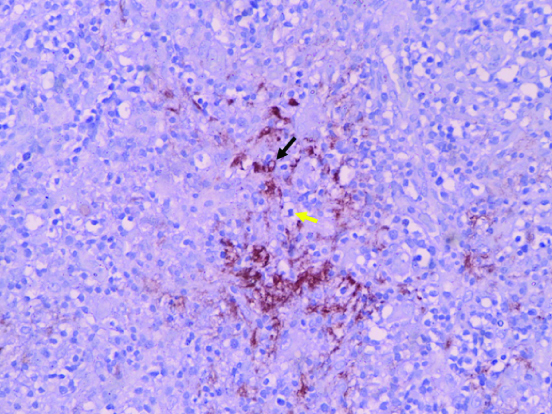
Between the NPTCL groups, the two entities that were commonly confused on morphology were PTCL-NOS and AITL since anaplasia in the ALCL entities is literally unmistakeable. In fact, epithelial and germ cell malignancies formed morphologic differentials of few ALCL cases. Classic histologic features such as a marked increase in vascularity (high endothelial venules), perivascular clear cells and patchy collections of plasma cells more often turned out to be AITL on IHC. The two small cell variant of ALCL, were confused to be PTCL-NOS on independent H&E slide review. IHC, eventually had fetched the diagnosis.
It was observed that no individual histomorphological parameter (see materials and method) was associated strongly with prognosis, either adversely or favourably. Thus, IHC is a reassuring adjunct. Still, the importance of a diligent morphologic study of a thin H&E lymph node section cannot be underscored since morphology, in itself, often gives away the diagnosis and also dictates the prudent initial panel of IHC markers in low resource settings.
For the sake of completion, it may be mentioned that while many prognostic factors and markers of aggressiveness exist, the present study did not dwell or intend to comment on the same given the lack of appropriate technology, complete clinical workup and lack of rigid follow-up in most patients [1]. Three scoring systems for prognostication of PTCL-NOS are worth mentioning. The Prognostic Index for peripheral T cell lymphomas (PIT) system includes Bone Marrow involvement, age, and performance status and serum Lactate Dehydrogenase levels [35]. The modified PIT or mPIT score additionally uses degree of Ki-67 positivity by IHC as an extra parameter [36]. Also, in 2018, the International T Cell Project Network has proposed a new score based on serum albumin, performance status, stage and absolute neutrophil count to identify those with very unfavourable outcomes [37]. The above mentioned references may be looked up by interested readers for an elaborate discussion.
To summarise, a good appraisal of morphology was often sufficient in providing a correct diagnosis. Demographic data, clinical features and radiological findings were not associated with any particular histological subtype but are important for staging of the disease. There was a definite increase in incidence of this group of nodal T-lymphoid neoplasms in our South Indian study population. A majority of them presented with advanced stage disease concordant with known literature. In fact, PTCL-NOS and AITL were statistically associated with Stage IV disease.
Limitation
Lack of adequate follow-up data acted as a roadblock towards a complete assessment of survival statistics as it was available for only 30 out of 80 patients included in the study. A skewed distribution of patients between subgroups (for e.g., 41 patients in PTCL-NOS vs. eight patients of AITL) may have led to under- or overrepresentation of certain clinico-radiological features or pathological findings among the disease subgroups esp. AITL and ALK-negative ALCL. This is because minor changes in numerator (for example, number of patients with any histological parameter such as necrosis in a node) may present as large shifts in percentage when denominator (i.e., total number of patients) is a small subgroup such as AITL.
Future Recommendations
Morphological awareness of the entity is crucial and pathologists need to be aware of the subtle clues such as reactive eosinophils and polymorphic population of lymphoma cells. Immunohistochemically, these lymphomas can be further well characterised. Attempts may be made to study various markers not only for the sake of diagnosis but also in relation to prognosis and treatment. For example, Brentuximab vedotin, an anti-CD30 monoclonal antibody is already available for use in Hodgkin lymphoma and ALCL [38]. Also, large scale epidemiologic studies need to be undertaken to ascertain a possible viral aetiology in these lymphomas.
Conclusion
As per the present study, except Anaplastic Large Cell Lymphoma (ALCL), Nodal Peripheral T-Cell Lymphomas (NPTCL) represent a high stage disease of the old. Pathological evaluation is challenging in view of the wide histomorphological variability. Given the fact that NPTCL is not a clinical diagnosis, awareness regarding this entity and its histopathological heterogeneity among pathologists is imperative. The increased incidence of these lymphomas in this study may represent a true geographic variation and further epidemiological or microbiological studies would do good to explore the genesis of these lymphomas.
*Abd.=Abdominal symptoms; †Resp=Respiratory symptoms
*a=Absent; †I=Localised; ‡g=generalised; §C=Cervical; ||A=Axillary; **I=Inguinal; ††IA=Intra-abdominal; ‡‡IT=Intra-thoracic; §§S=Supraclavicular; |||HSM=Hepatosplenomegaly
*PI=Perinodal infiltration; †DE=Diffuse effacement; ‡IV=Increased vascularity; §Mitosis=Classified as high or low on the basis of 10 mitotic figures/10 high power fields; ||Variant=Variant morphological patterns; PTCL-NOS=5 Lennerts; 2 Interfollicular; 1 Follicular; ALCL-ALK+=2 null cell, 1 small cell, 1 lymphohistiocytic, 1 Nodular sclerosis like; ALCL-ALK-=1 small cell; 1 Nodular sclerosis like; **E=Eosinophils; ††H=Histiocytes; ‡‡L=Lymphocyte; §§P=Plasma cells
*The numerical values for follow-up duration and current status of patients are calculated keeping in mind a total of 30 patients that include 13,9,4 and 4 patients of PTCL-NOS, ALK-positive ALCL, ALK-negative ALCL and AITL respectively for whom the data was available while Clinical Stage is calculated for 41,23,8 and 8 patients of PTCL-NOS, ALK-positive ALCL, ALK-negative ALCL and AITL; † the values reflect the case fatality rate and represent the mortality risk of the disease