Cisplatin is one of the important anticancer drugs widely used in the treatment of cancers. Since cisplatin gets accumulated about 5 times the serum concentration in renal tubular epithelial cells and excreted through kidney, the chances for nephrotoxicity is higher during cisplatin therapy [1]. Even though dose dependent nephrotoxicity is the dose limiting side effect of cisplatin, it remains as a standard component of treatment regimen for most of the solid organ cancers such as cervical, squamous cell carcinoma of head-neck, ovarian, testicular, bladder, and lung cancers. Despite the recovery of renal toxicity occur over a period of four weeks, there are some reports on lack of recovery, progressive and permanent nephrotoxicity with successive treatment [2,3]. Risk factors for cisplatin nephrotoxicity are dose, frequency, cumulative dose, older age, female gender, smoking, hypoalbuminemia and pre-existing renal impairment [4,5]. In addition to inflammatory response, cisplatin causes damage to mitochondrial and nuclear DNA and production of Reactive Oxygen Species (ROS) lead to activation of both mitochondrial and non-mitochondrial apoptosis and necrosis of renal epithelial cells. Anticancer mechanism of cisplatin is responsible for the cisplatin induced renal toxicity. Therefore, strategies intended to reduce cisplatin renal injury may have the unintended consequence of reducing the anti-tumour actions of cisplatin. Thus, the design of preventive strategies must carefully consider this risk [6].
Chemoprotective agents are those agents used with chemotherapy (anticancer drugs) to protect the body from or minimize the side effects of the chemotherapy. FDA approved chemoprotective agents include amifostine (protects renal tissue), dexrazoxane (protect heart) and mesna (protects bladder). Even though these drugs do not eliminate side effects in general, they protect the body from some potentially serious side effects. Since these drugs also have their own adverse effects, they are used only in special circumstances, when benefit clearly is greater than the risk. An ideal chemoprotective agent should reduce systemic toxicity without impairing anti-tumour response of the chemotherapeutic agent. In case of cisplatin, a nephroprotective agent is expected to reduce the renal toxicity without impairing the anticancer efficacy of cisplatin. Current research focuses on identifying compounds which reduce renal accumulation of cisplatin or compounds with antioxidants, anti-apoptosis and anti-inflammatory properties to reduce cisplatin induced nephrotoxicity [6].
Thus, the objective of the current study was to compare the cytoprotective activity of crude aqueous extract and fractions of whole VC plant in normal HEK293 cells and cancer HELA cell lines against cisplatin induced cytotoxicity.
Materials and Methods
This was an invitro study conducted at Manipal Academy of Higher Education, Manipal, Karnataka, India during August-October 2017.
Collection of Plant and Authentication
Vernonia cinerea whole plant was collected from Manipal University campus and authenticated by Dr. KN Sunil Kumar, Taxonomy Expert from Department of Pharmacognosy, SDM Centre for Research in Ayurveda and Allied Sciences, Udupi, Karnataka and the Voucher specimen was preserved (no. 604.15042404) for future reference.
Crude Aqueous Extract (CAE)
The CAE of the whole plant was prepared by soaking 500 g of powdered sample in 3.5 L of distilled water for 7 days by cold maceration method [10]. The extract was then filtered using Whatmann filter paper. Again 3.5 L of fresh distilled water was added to the plant material to obtain further extract as mentioned above and the procedure was repeated three times using the same plant materials. The extract was then dried by keeping in water bath, followed by desiccator until there was no further weight loss. The percentage yield of CAE was calculated using the formula; (weight of extract/weight of plant material) X 100.
Fractionation of Crude Aqueous Extract
The CAE was further fractionated as follows; 20g of aqueous extract was dissolved in 100 mL (5 times of extract) of distilled water and taken in a separating funnel (1000 mL capacity). The extract was separated into fractions of varying polarity using solvents like petroleum ether, chloroform, ethyl acetate, n-butanol, ethanol and water. Each solvent was (300 mL) added in the separating funnel, shaked vigorously, allowed for separation in stand. Then the solvent was filtered. This procedure was repeated until clear solvent was obtained. The percentage yield of the fractions was calculated using the formula; (weight of fraction/weight of crude aqueous extract) X100.
Phytochemical Screening of Crude Aqueous Extract and its Fractions
The phytochemical examination of aqueous extract of Vernonia cinerea was performed in our pharmacognosy lab by following the standard methods [11,12].
Tests for Alkaloids
Dragendorff’s test: A 500 mg of extract dissolved in alcohol was shaken well with a few drops of acetic acid and Dragendroff’s reagent. An orange red precipitate formed indicated the presence of alkaloids.
Wagner’s test: To a 500 mg of extract dissolved in acetic acid, a few drops of Wagner’s reagent was added. A reddish brown precipitate formed indicated the presence of alkaloids.
Mayer’s test: To a 500 mg of extract dissolved in acetic acid, a few drops of Mayer’s reagent was added. A dull white precipitate formed indicated the presence of alkaloids.
Hager’s test: To a 500 mg of extract dissolved in acetic acid, 3 mL of Hager’s reagent was added; the formation of yellow precipitate indicated the presence of alkaloids.
Tests for Carbohydrates
Molisch’s test: To 500 mg of extract, 1 mL of α-naphthol solution and concentrated sulphuric acid were added along the sides of test tube. Violet colour formed at the junction of the two liquids indicated the presence of carbohydrates.
Fehling’s test: A 500 mg of extract was mixed with equal quantities of Fehling’s solution A and B. The mixture was warmed on a water bath. The formation of a brick red precipitate indicated the presence of carbohydrates.
Benedict’s test: To 5 mL of Benedict’s reagent, a 500 mg of extract was added, and boiled for two minutes and cooled. Formation of a red precipitate indicated the presence of carbohydrates.
Test for Steroids
Libermann-Burchard test: To a 500 mg of extract dissolved in chloroform, 1 mL of acetic acid and 1 mL of acetic anhydride were added, then heated on a water bath and cooled. Few drops of concentrated sulphuric acid were added along the sides of the test tube. Appearance of bluish green colour indicated the presence of steroids.
Salkowski test: To 500 mg of extract was dissolved in chloroform and equal volume of concentrated sulphuric acid was added. Formation of bluish red to cherry red colour in chloroform layer and green fluorescence in the acid layer indicated the presence of steroids.
Test for Saponins
To a 500 mg of extract, distilled water was added and shaken. Stable froth formation indicated the presence of saponin.
Test for Tannins
To the 500 mg of extract, a few drops of dilute solution of ferric chloride was added, formation of dark blue colour showed the presence of tannins.
Test for Flavonoids
Shinoda’s Test: To the 500 mg of extract in alcohol, a few magnesium turnings and few drops of concentrated hydrochloric acid were added and heated on a water bath. Formation of red to pink colour indicated the presence of flavonoids.
Test for Phenol
To the 500 mg of extract in alcohol, added two drops of alcoholic ferric chloride. Formation of blue to blue black colour indicated the presence of phenol.
Test for Coumarins
To the 500 mg of extract in alcohol, a few drops of 2 N sodium hydroxide solution were added. Dark yellow colour formation indicated the presence of coumarins.
Test for Triterpenoids
The 500 mg of extract was warmed with tin bits and few drops of thionyl chloride. Formation of pink colour indicated the presence of triterpenoids.
Test for Carboxylic Acid
A 500 mg of extract dissolved in water is treated with sodium bicarbonate. Brisk effervescence indicated the presence of carboxylic acid.
Test for Resin
A 500 mg of the sample was mixed with water and acetone. Turbidity indicated the presence of resin.
Test for Quinine
A 500 mg of extract in alcohol was treated with 0.5% of sodium hydroxide. Deep coloration like pink, purple or red indicated the presence of quinine.
Cell Culture
Cell culture supplies were obtained from Invitrogen Pvt., LTD., (Bangalore). Chemicals were obtained from Sigma Chemical Co. (St. Louis, MO, USA). HEK293 cells, and HELA cell lines were obtained from National Centre For Cell Science, Pune, India. When the cell confluence was about 80%, subculture was done. For sub culturing, the cells were seeded at 104 cells/cm2 in 9 mL volume six-well plates using Dulbecco’s Modified Eagle’s Medium (DMEM) containing L-glutamine, sodium pyruvate, sodium bicarbonate and glucose (4500 mg/L) supplemented with 10% Fetal Bovine Serum (FBS). Antibiotic gentamycin was added and maintained in a humidified atmosphere of 95% air/ 5% CO2 at 37°C. Trypsin was used as dissociation reagent.
Calculation of IC50 of Cisplatin in Two Cell Lines
The cells were harvested from the medium and resuspended to a final concentration of 10,000 cells/100 μL (for 24 hrs incubation) and 5,000 μL (for 48 hrs incubation) of fresh medium containing 10% FBS. Cell suspensions (100 μL) were dispensed into individual wells of a 96-well tissue culture plate with a lid (Falcon, Oxnard, CA). The cells were incubated at 95% air/ 5% CO2 at 37°C temperature for 24 hours for attachment to the 96 well tissue culture plate. Triplicate was set-up for each experimental group. In the respective plates, blank column was kept containing medium alone, control column containing cells but no drugs, cisplatin control column containing cells with cisplatin in different doses (10, 20, 50, 100, 200, 500 μg/mL). Cell lines were incubated with cisplatin for 48 hours.
Viable cells were determined by MTT reduction assay for the cisplatin control and different drug doses. MTT values were observed at absorbance of 540 nm. The % cell death was calculated by using the formula: {(cisplatin-control)/control}×100.
Dose-response (% cell death) curve was plotted using MS excel. The linear trendline with formula (Y=mX+c) was drawn, where Y is the % cell death, X is the dose of cisplatin, m is the slope and c is the intercept. The IC50 value of cisplatin was calculated using the formula; x=(50-c)/m.
MTT Assay for the Herbal Extract and Fraction
The cells were harvested from the medium and resuspended to a final concentration of 10,000 cells/100 μL (for 24 hrs incubation at 95% air /5% CO2 at 37°C temperature) and 5,000 μL (for 48 hrs incubation) of fresh medium containing 10% FBS. Cell suspensions (100 μL) were dispensed into individual wells of a 96-well tissue culture plate with a lid (Falcon, Oxnard, CA). These were kept for attachment to the 96-well tissue culture plate for a period of 24 hours. Triplicate was done for each well. In the respective plates, blank column was kept containing medium alone, control column containing cells but no drugs, cisplatin control column and test columns containing cells with cisplatin in its IC50 dose. The IC50 value of cisplatin was derived from our lab (described previously). CAE, BF and AF at different concentrations (50 μg, 100 μg, 200 μg, 500 μg, 1000 μg, 2000 μg) were then added (100 μL) to cell suspensions of test columns. Cell lines were incubated with the drugs for 48 hours.
Viable cell growth was determined by MTT reduction assay for the cisplatin control and herbal extract and fractions, in triplicate. MTT values were observed at absorbance of 540 nm.
The % cell death was calculated by using the formula:
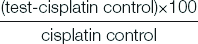
The % cell viability was calculated by the formula: 100-% cell death and the dose-response (% cell viability) graph was plotted in MS excel. The % improvement in cell viability due to the extract/fractions was calculated by the formula: {(test-cisplatin control) /cisplatin control} X100.
Statistical Analysis
The % cell viability data was expressed in mean±SD. The data was analysed by SPSS 16.0 version using One-Way ANOVA by keeping p<0.05 as significant. Values of test groups were compared with cisplatin control group by Dunnett’s (2-sided) post-hoc test.
Results
Extract and Fractions Yield
The CAE was fractioned only in n-butanol (Butanol Fraction-BF) and not dissolved in all other solvents. Thus, the remaining fraction after BF was named as Aqueous Fraction (AF). The percentage yield of CAE, BF and AF were 8 %, 18.6% and 81.4% w/w respectively.
Phytochemical Analysis
The qualitative phytochemical investigation of the aqueous extract and its BF of Vernonia cinerea whole plant showed the presence of carbohydrate, steroid and saponin, whereas aqueous fraction contains only carbohydrate and steroid, which lacked saponins.
IC50 Value of Cisplatin
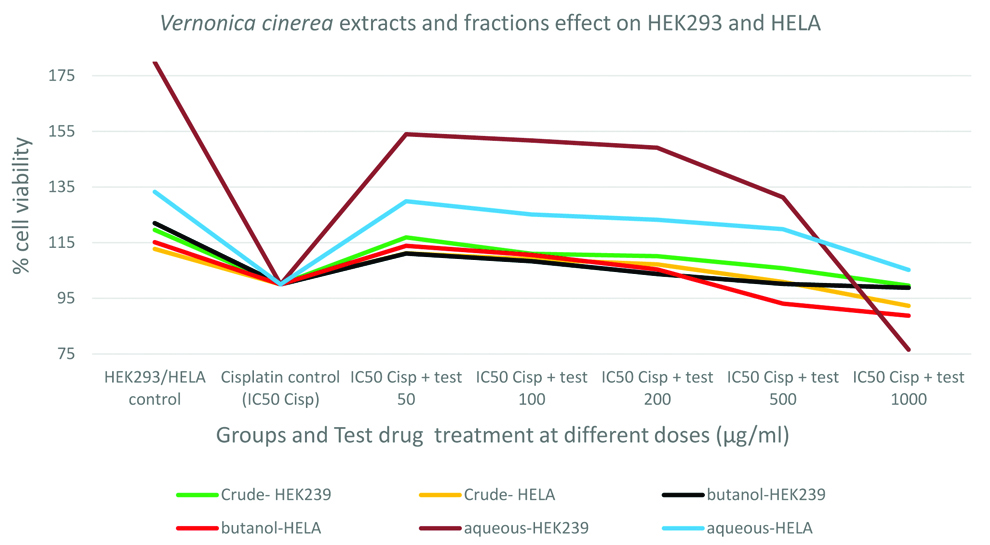
The IC50 value of cisplatin in HEK293 cells and HeLa cells are 4.72 μg/mL and 6.29 μg/mL respectively. For further cytoprotective studies, these doses were used to induce cisplatin induced cytotoxicity [Table/Fig-1].
Mean % cytotoxicity and IC50 of cisplatin in normal and cancer cell lines.
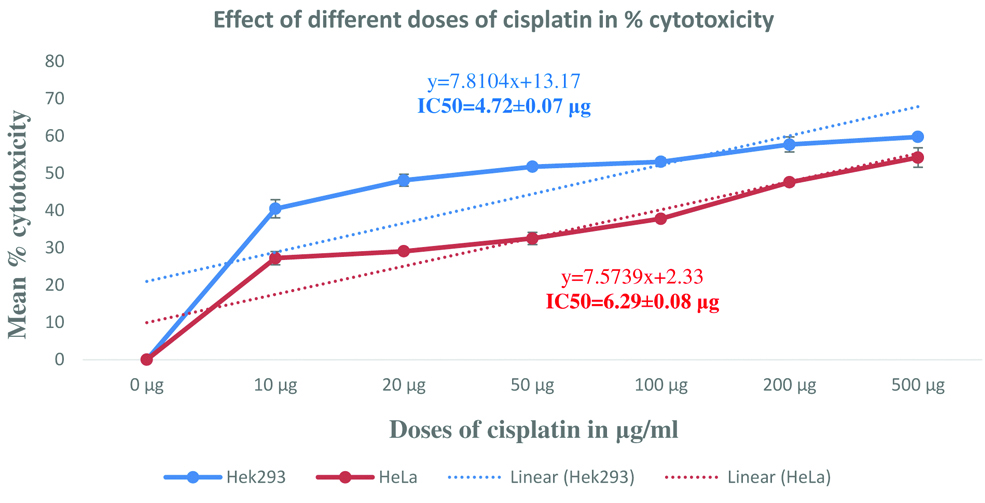
Protective effect of CAE/Fraction in human embryonic kidney (HEK293) cells: As shown in the [Table/Fig-2], cisplatin exposure had reduced the HEK293 cell viability by about 20%, 22% and 80% before treating the cells with CAE, BF and AF respectively. The CAE and AF showed significant (p<0.001) protective (improvement in % cell viability) activity in HEK293 cells against cisplatin. The protective activity was maximum at lower doses and it was lost at higher doses. Crude extract and aqueous fraction had shown maximum protection (improvement in cell viability) by 17% and 54% respectively. Even though the BF showed improvement in cell viability by 11% after cisplatin exposure, it was statistically insignificant. The aqueous fraction had shown 2.8 times higher cytoprotection than the CAE against cisplatin [Table/Fig-2].
Protective effect of crude aqueous extract and its fractions in human embryonic kidney (HEK293) cells compared with cisplatin treated cell group.
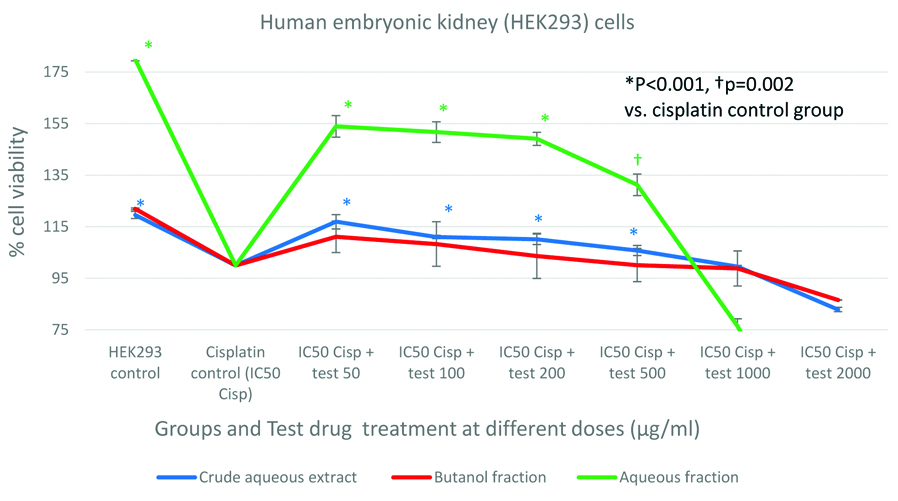
Protective effect of CAE/Fraction in human cervix epitheloid carcinoma (HELA) cells: Cisplatin exposure has reduced the HELA cell viability about 12%, 15% and 33% before treating the cells with herbal extract/fractions. All three test compounds; CAE, BF and AF had shown significant (p<0.001) protective (improved % cell viability) activity in HELA cells against cisplatin. The protective activity was maximum at lower doses and lost at higher doses. It was observed that the AF had shown highest cytoprotection (30% improvement in cell viability) than the CAE against cisplatin (11% improvement in cell viability) and BF (13% improvement in cell viability). In addition, the AF had shown 1.73 times higher cytoprotection than the CAE and 1.55 times higher cytoprotection than BF against cisplatin [Table/Fig-3].
Protective effect of crude aqueous extract and its fractions in human cervix epitheloid carcinoma (HELA) cells compared to cisplatin treated cell group.
Comparison of protective effect of CAE, BF and AF among normal and cancer cells: Among all the three test compounds, the AF had exhibited higher cytoprotection in HEK293 cells against cisplatin, followed by in HELA cells. The AF had shown 80% higher cytoprotection (54% improvement in cell viability) towards normal HEK293 cells than HELA cancer cells (30% improvement in cell viability) against cisplatin induced toxicity. This clearly suggests that the AF could be having compounds specific for the protection of normal renal cells. CAE of whole plant and its BF had lesser cytoprotective activity than AF [Table/Fig-4].
Comparison of protective effect of crude aqueous extract, butanol fraction and aqueous fraction among normal Human Embryonic Kidney (HEK293) and Human Cervix Epitheloid Carcinoma (HELA) cancer cells.
Discussion
VC is being used in Siddha Medicine to alleviate metal drugs induced toxicities. Cisplatin is an inorganic anticancer agent that specifically damages kidney. This study was aimed to compare the protective activity of the herbal extract and its fractions in normal kidney cells as well as cancer cells against cisplatin induced cytotoxicity. In our study, we used decoction (CAE) of VC and its two fractions to find out its cytoprotective activity against cisplatin induced cell toxicity in a normal kidney cell line (HEK293) and a cancer (HELA) cell line.
The cytoprotective activity of the extract and fractions were observed only in low doses and at higher doses the protective activity declined. This declining of protective effect at higher doses might be due to the presence of cytotoxic compounds such as vernolide-A, that could exhibit the cytotoxic effect when sufficient concentration is added while increasing the dose. Crude extract and aqueous fraction had shown maximum cytoprotection (17% and 54% respectively) in normal kidney cells, whereas the AF showed 2.8 times higher cytoprotection than the CAE against cisplatin. The AF had shown 80% higher cytoprotection (54% improvement in cell viability) towards normal HEK293 cells than HELA cancer cells (30% improvement in cell viability) against cisplatin induced toxicity.
Different extracts of VC have been proved for analgesic, anti-inflammatory [13], antipyretic [14], antifungal [15], antidiarrheal [16], antioxidant properties and its ability to down regulate proinflammmatory cytokines and its gene expression [17]. Ethanol extract of whole plant showed significant antihyperglycaemic activity in alloxan induced diabetic mice [18] and also ameliorated symptoms of haloperidol induced catalepsy in rats [19]. Ethanol and chloroform extracts of aerial parts of VC showed good anticancer activity against Dalton’s Ascitic Lymphoma [20]. Bioassay guided fractionation of ethanol extract lead to isolation of vernolide-A, which is the active principle for its anticancer activity [21]. It inhibited lung metastasis of melanoma cells in mice and increased life span of mice [22].
Several studies have proved the antioxidant property of VC in different animal models [23-25]. VC whole plant powder protected liver against carbon tetrachloride induced liver damage in rats [26,27]. Intraperitoneal administration of methanol extract in mice showed a significant protective activity without compromising the chemotherapeutic efficacy of cyclophosphamide and gamma radiation. In these studies, extract increased WBC count, bone marrow cellularity and weight of lymphoid organs. It also reduced the small intestine damage and bone marrow DNA damage caused by cyclophosphamide [28,29]. The cytoprotective activity of aqueous extract against nicotine induced damage in human umbilical vein endothelial cells has been already reported [30]. Hexane extract of trunk possessed immunomodulatory effect by significantly reducing IL-6 level via an inhibition of NF-KB nuclear translocation in isolated peripheral blood mononuclear cells of healthy volunteers [30].
Another comparative study was done with hexane, chloroform, methanol and aqueous extract of the plant. Agarose gel electrophoresis demonstrated the DNA protective activity of aqueous extract against UV-photolysis of H2O2 induced pBR322 plasmid DNA damage, which suggests the chemoprotective potential of VC aqueous extract in cytotoxicity [31]. The preliminary study on protective activity of aerial parts of petroleum ether, ethyl acetate and ethanol extracts of VC in cisplatin-induced nephrotoxicity was reported in rats by Sreedevi A et al., [32]. Different authors reported that different activities (cytoprotective as well as cytotoxic) for the same extract. For example, some authors showed that ethanol extract has cytotoxic effect and others cytoprotective activity. Our study suggests the protective potential of AF obtained from CAE on normal renal cells than cancer cell.
Ten compounds with nerve growth factor inducing activity have been isolated and characterized from this plant [33,34]. This whole plant contains variety of sterols, aliphatic acid, terpenoids, flavonoids, saponins, tannins, and fatty oil [35]. The preliminary analysis of aqueous extract of aerial parts showed the presence of flavonoids, saponins and tannins [36]. The AF contains only carbohydrate and steroids. The major phytoconstituents of the plant includes lupeol, lupeol acetate, luteolin-7-O-glucoside, stigmasterol-β-D-glucopyranoside, stigmasterol and dotriacontanoic acid. Other minor phytochemicals are luteolin, luteolin-7-O-glucuronide, diosmetin, iso-orientin, chrysoeriol, lupeol palmitate, β-amyrin, β-amyrin acetate, α-amyrin, α-amyrin acetate, α-amyrin palmitate, δ-amyrin acetate, 3β-acetoxyurs-13(18)-ene, 3β-acetoxyurs-19-ene, 24-hydroxy taraxer-14-ene, vernolide A, vernolide B, stigmast-5,17(20)-dien-3β-ol, campersterol, α-spinasterol, β-sitosterol, sitosterol and 26-methylheltacosanoic acid [37].
Aqueous extract of whole plant stimulated presynaptic cholinergic nerve endings of rat duodenal smooth muscle to produce spasmodic response in isolated duodenum [38]. Aqueous extract also improves insulin-resistance in high-fat diet-induced obese mice [39]. When aqueous extract was given to smokers, it reduced smoking rate [40]. In our study, crude aqueous extract was used that exhibited cytoprotective activity against cisplatin induced cell damage. The phytochemicals responsible for the nephroprotective action is yet to be studied along with mechanism. The cell line studies can’t be directly extrapolated on the effects in whole body system, thus the study must be repeated in suitable animal models or in cancer patients.
Limitation
Traditionally, the herbal decoction (kashayam) was prepared by boiling the herb in water, which is used by traditional practitioners. In this study, we prepared the CAE in cold maceration method, in which we did not heat. There may be chemical changes while applying heat during the traditional extraction procedure, which was not followed in this study. This is one of the limitations of the study. The extract taken by hot percolation method could be compared for cytoprotective activity. Further isolation of phytochemicals and their cytoprotective properties to be compared.
Conclusion
Neichitti kashayam (CAE of Vernonia cinerea) and its AF have shown protective activity against cisplatin induced renal cell damage. This study has created the scientific basis for using this herbal decoction (CAE) against metal toxicities by Siddha physicians. The AF has the potential to protect specifically normal renal cells than cancer cells against cisplatin damage and hence it could be the potential candidate for nephroprotective drug discovery. This fraction or the lead compound may be the potent drug to protect kidney among cancer patients who receive cisplatin therapy. However, further study should be carried out to know how this fraction behaves in-vivo, the dose regimen for complete protection, the lead compound, and the molecular mechanism of cytoprotective action against cisplatin induced renal toxicity.