Consistent Level IIa Node as a Surgical Landmark for Identification of Spinal Accessory Nerve
Vidita Powle1, Vishal Yadav2, Sushma Mehta3, Abhishek Ghosh4
1 Fellow, Department of Head and Neck Surgical Oncology, Mazumdar Shaw Cancer Center, Bangalore, Karnataka, India.
2 Assistant Consultant, Department of Head and Neck Surgical Oncology, Rajiv Gandhi Cancer Institute, Delhi, India.
3 Fellow, Department of Head and Neck Surgical Oncology, Mazumdar Shaw Cancer Center, Bangalore, Karnataka, India.
4 Senior Resident, Department of Head and Neck Surgical Oncology, Homi Bhabha Cancer Hospital, Varanasi, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Abhishek Ghosh, Old Loco Colony, Shivpurwa Homi Bhabha Cancer Hospital, Varanasi, Uttar Pradesh, India.
E-mail: 13abhishekghosh@gmail.com
Introduction
Treatment of Oral Squamous Cell Carcinoma (SCC) requires a composite resection of the lesion and elective/therapeutic neck dissection, whenever a surgical option is being considered as a form of treatment. During neck dissection, one of the critical structures to be preserved is the Spinal Accessory Nerve (SAN). In our experience of operating on N+ necks for selective neck dissections, we found the presence of a constant lymph-node at the level of hyoid bone/digastric muscle tendon lateral to the internal jugular vein (level IIa). Direct deeper dissection exactly beneath this node led to the identification of the SAN.
Aim
The aim of the study was to ascertain level IIa lymph node as a landmark for identification of SAN, during neck dissection procedures.
Materials and Methods
In this prospective study, 100 consecutive selective neck dissection procedures done for patients with radiological N+ necks with resectable Oral SCC were included.
Results
In 96% of cases identification of SAN was done with the technique used alone.
Conclusion
The purpose of this study was to establish this technique as a standard of care either alone or in adjunct with other established landmarks for identification of SAN, by trainees as has been a long established practice in our department. We even have a separate nomenclature for it, the ‘Trainee Node.’
Neck dissection, Oral SCC, Shoulder syndrome
Introduction
Treatment of Oral Squamous Cell carcinoma, for a resectable disease includes composite resection of the lesion and elective/therapeutic neck dissection, followed by appropriate reconstruction and adjuvant therapy as per post-operative histopathology report. Preservation of the Spinal Accessory Nerve (SAN) is one of the critical steps during neck dissection, if it is not directly involved by the disease per se. The nerve’s careful identification, subsequent handling and preservation is mandatory to avoid the drooping shoulder syndrome, characterised by serious limitations of function of the shoulder girdle [1].
Despite the number of methods described to aid in location of the nerve, it remains a difficult and critical step to be performed by a head and neck surgeon. Our institutional experience has been that the presence of multiple branches of the cervical plexus often complicates this step and they are sometimes mistaken for the main nerve trunk, especially if the patient has a short fat neck, in which case proper neck extension is not achievable thus restricting access to the area.
While operating on N+ necks for selective neck dissections for Oral Squamous Cell Carcinoma, we found the presence of a constant lymph-node at the level of hyoid bone/digastric muscle tendon, lateral to the internal jugular vein (level IIa). Directly beneath lies the SAN, which can be identified by deeper dissection. This technique, to the best of authors’ knowledge, has never been described or studied in literature before. Thus, this study was designed to ascertain this lymph node as a landmark, which could serve as a pilot for future detailed investigations regarding the presence and implications of this node.
Materials and Methods
This is a prospective study, in which 100 consecutive selective neck dissections done for in 91 patients (82 patients underwent ipsilateral neck dissection and 9 patients underwent bilateral neck dissection) with radiological N+ necks with resectable Oral Squamous Cell Carcinoma presenting at our Head and Neck Surgical Oncology Department between January 2018 to March 2018 were included in the study. Bilateral neck dissections for the purpose of this study have been considered as two neck dissections.
Inclusion Criteria
Treatment naïve patients with resectable oral squamous cell carcinoma.
Radiologically or clinically N+ neck (N1, N2a, N2b, N2c) -AJCC 8th Edition [2].
Exclusion Criteria
Non-squamous cell pathology/ non oral cavity pathology.
Radiologically or clinically N 3 (AJCC 8th Edition) nodal stage [2].
Ethical clearance was not sought for this pilot study, as this study intended to be a modification of already well-established surgical technique, which has been in practice at our centre, prior to this designed study.
Technique
Sub-platysmal flaps were raised in the standard manner. The anterior border of the Sternocleidomastoid (SCM) muscle was delineated. The SCM was divested of its fascia. The avascular plane, between the SCM and Internal Jugular Vein (IJV), was opened by sharp dissection [1]. Here on according to the technique practiced at our institution, the posterior belly of the digastric muscle was identified and retracted. At this point the level of the hyoid bone was identified and a node parallel to this level lateral to the IJV was identified. The loose areolar tissue on the inner aspect of the SCM, which separates it from the internal jugular vein, was further opened up at the level of the identified node and the Spinal Accessory Nerve (SAN) was identified directly beneath the node at its entry point into the SCM [Table/Fig-1a-c].
a) Blue arrow-retracted SCM, black arrow-retracted IJV, White arrow-undissected lymph node lying above SAN in a left neck dissection. b) Black arrow-retracted IJV, blue arrow-retracted SCM, white arrow-babcock forceps holding dissected node lying above SAN, pointed by Addison forceps. Right Neck Dissection. c) Black arrow-IJV, blue arrow-retracted SCM, white arrow-node lying above SAN, yellow arrow-digastric tendon at the level of hyoid bone. Left neck dissection.
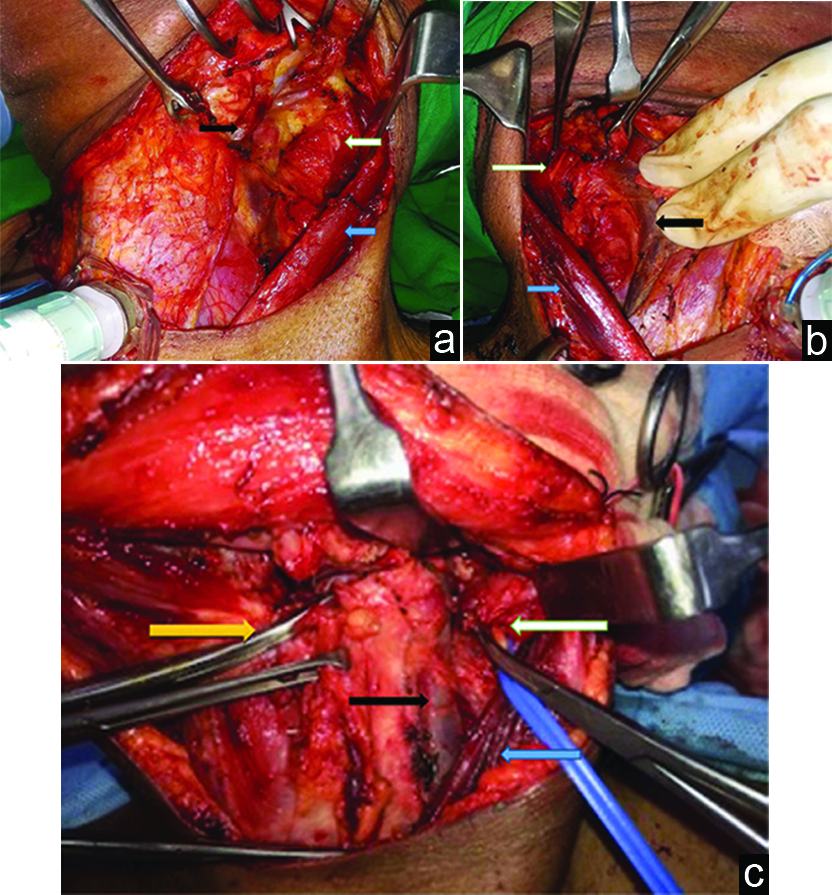
Results
In this study, 91 consecutive patients with N+ neck, underwent 100 selective neck dissection. 82 patients underwent ipsilateral neck dissection and 9 patients underwent bilateral neck dissection [Table/Fig-1,2]. For the purpose of this study bilateral neck dissection has been considered as two neck dissections (82 ipsilateral neck dissections+9 bilateral neck dissections=total 100 neck dissections). Strictly following the above described technique alone, we were able to identify the SAN near its entry point into the SCM in 96 neck dissection procedures. Except for in four neck dissections were a nodal conglomerate was found to be surrounding the nerve and the SAN had to be sacrificed for oncological safety. Pre-operative scans, for these four patients were suggestive of the presence of nodal conglomerate at level II, however it is not possible to identify the course of the nerve in a Contrast Enhanced Computer Tomography (CECT) image, which is the more common pre-operative imaging modality used. Identification of the nerve although possible on Magnetic Resonance Imaging (MRI), is not a routine practice and MRI is not advised to the patient just for identification of the nerve unless otherwise indicated.
Gender distribution and type of neck dissection procedures.
| No. of Patients | Neck Dissections |
|---|
| Ipsilateral | Bilateral |
|---|
| Gender | Male | 76 | 69 | 7 |
| Female | 15 | 13 | 2 |
| Total | | 91 | 82 | 9 |
In our experience, despite the presence of multiple nodes in level II, it is only rarely that the nerve passes through a nodal conglomerate and that only becomes evident on the operating table during dissection. These 4 neck dissections had been staged N2b clinically with the primary being T4a buccal mucosa OSCC [Table/Fig-3].
Total number of cases of oral squamous cell cancer, primary site and nodal staging.
| Serial no. | Oral Cavity Sub-site | No. of patients | Neck Dissections |
|---|
| Ipsilateral | Bilateral |
|---|
| N1 | N2a | N2b | N2c |
|---|
| 1. | Buccal Mucosa | 52 | 18 | 7 | 27 | |
| 2. | Tongue | 20 | 5 | 3 | 7 | 5 |
| 3. | Floor of Mouth | 5 | | | 1 | 4 |
| 4. | Lower Alveolus/Gingivo- Buccal Sulcus | 14 | 4 | 2 | 8 | |
| Total | 91 | 27 | 12 | 43 | 9 |
| 82 |
Discussion
Identification of SAN is an important step in neck dissection. Its anatomic implication with regards to neck dissection is that it delineates level IIa and IIb in the neck. Its intraoperative identification serves two purposes:
Segregation of lymph nodal groups, IIa and IIb.
Prevents iatrogenic injury to the nerve itself, thus avoiding postoperative shoulder syndrome [3].
Its rapid identification while preserving its nerve sheath and innervation to Sternocleidomastoid (SCM) and Trapezius is vital for post-operative quality of life of the patient [4]. The most consistent landmarks for its identification are greater auricular nerve at erb’s point and its entry point into trapezius in the lower neck [5]. With consistent use of Selective Neck Dissections (SND) in N+ necks and Super Selective Neck Dissections (SSND) in salvage settings, the need for identification of SAN at higher levels of neck by simpler means in anterograde manner is felt, however only few papers in literature discuss this issue [6,7].
The method described in this study requires identification of a level IIa lymph node, which has been consistently found at the level of the hyoid bone, lateral to IJV following retraction of SCM, in the avascular plane. We were able to identify the SAN by direct deeper dissection to this node in 96 out of 100 neck dissection procedures. In the remaining four cases the nerve was found to be traversing through a nodal conglomerate in this region and had to be sacrificed for oncological safety.
In the superior neck, the transverse process of the (C1) has been described in the literature as the key landmark [8,9]. However, palpatory identification of the transverse process with intact level IIb is difficult and often not possible in short fat necks where in-adequate extension of the neck is often not achievable. In contrast it is much easier to identify the node at this level, which in our experience was found to be consistently lying over the SAN at the level of hyoid bone.
Limitation
The small sample size inherently limits any conclusion drawn from this study to be generalised. Thus this study can be treated as a pilot study. Further studies with larger sample size are required to prove level IIa node as consistent landmark for SAN identification as lymph nodes in general are not considered to be consistent in their location. This study only includes N+ necks and further investigations are warranted to identify the presence or absence of this node in clinically N0 necks.
Conclusion
In our search of the published literature, while preparing the manuscript of this study, we have not come across mention of any such technique involving the constant presence of this node in N+ necks while doing Selective Neck Dissection (I-IV) used to identify the SAN. The purpose of this study was to establish this technique as a standard operating procedure for identification of SAN We even have a separate nomenclature for it, the ‘Trainee Node.’
[1]. Chaukar DA, Pai A, D’Cruz AK, A technique to identify and preserve the spinal accessory nerve during neck dissectionJ Laryngol Otol 2006 120:494-96.10.1017/S002221510600087916772057 [Google Scholar] [CrossRef] [PubMed]
[2]. Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, The 8th edition of the AJCC cancer staging manual 2017 New YorkSpringer10.1007/978-3-319-40618-3_2 [Google Scholar] [CrossRef]
[3]. Nahum AM, Mulhally W, Manmoor L, A syndrome resulting from radical neck dissectionArch Otolaryngol 1961 74:42410.1001/archotol.1961.0074003043301114477989 [Google Scholar] [CrossRef] [PubMed]
[4]. Tatla T, lingam J, Majithia A, Clarke PM, Upper neck spinal accessory nerve identification during neck dissectionJ Laryngol Otol 2005 119:906-08.10.1258/00222150577478351116354345 [Google Scholar] [CrossRef] [PubMed]
[5]. Brandenberg JH, Lee CYS, The eleventh nerve in radical neck surgeryLaryngoscope 1981 91:1851-59.10.1288/00005537-198111000-00009 [Google Scholar] [CrossRef]
[6]. Eisele DW, Weymuller EA, Price JC, Spinal accessory nerve preservation during neck dissectionLaryngoscope 1991 101:433-35.10.1002/lary.1991.101.4.4331810288 [Google Scholar] [CrossRef] [PubMed]
[7]. Salgarelli AC, Landini B, nBellini P, Multinu A, Consolo U, Collini M, A simple method of identifying the spinal accessory nerve in modified radical neck dissection: anatomic study and clinical implications for resident trainingOral Maxillofac Surg 2009 13(2):69-72.10.1007/s10006-009-0152-x19277731 [Google Scholar] [CrossRef] [PubMed]
[8]. McMinn RMH, Head and neck and spine. In: McMinn RMH, edLast’s Anatomy. Regional and Applied 1994 9th ednLondonChurchill Livingstone:424-25. [Google Scholar]
[9]. Sheen TS, Chung TT, Snyderman CH, Transverse process of the atlas (C1)-an important surgical landmark of the upper neckHead Neck 1997 19:37-40.10.1002/(SICI)1097-0347(199701)19:1<37::AID-HED7<3.0.CO;2-W [Google Scholar] [CrossRef]