Intussusception is a serious medical condition in which a part of the alimentary tract invaginates into an adjacent segment and leads to intestinal obstruction commonly occurring in children younger than 2 years [1,2]. Moreover, intussusception was observed in 60% of children with <1 year of age and in 80% of the cases aged <2 years of age. Gender wise, the occurrence was found to be 3:1 for males and females respectively [3]. According to WHO report, in developed countries, the baseline incidence of intussusception is between 0.5-4.3 cases per 1,000 live births or 0.7-1.2 cases per 1,000 children aged <1 year [4]. The occurrence of intussusception in Indian infants appears to be lower than middle and high-income countries [5].
Rotavirus is an important pathogen causing diarrhea and is responsible for 150,000 deaths annually among Indian children <5 years of age [5]. This pathogen contributes significantly to incidence of intussusception as observed by several authors [6,7]. However, natural rotavirus infection is not a major contributor of intussusception in Indian infants [5]. Study on epidemiology of intussusception in Indian children is a prior requirement of rotavirus vaccination programme for decision making about use of new rotavirus vaccines. Accurate estimates of the incidence of intussusception are not available for most developing countries.
Therefore, a case-control study was conducted in a tertiary care hospital to examine the association of intussusception with natural rotavirus infection or vaccination in children aged less than five-year-old.
Materials and Methods
A case-control study was conducted at the Paediatrics ward of a tertiary care hospital with paediatric surgical facilities in Odisha during September 2016 to June 2018. Out of 2533 admitted children of <5 years of age, 17 cases with intussusception were taken, as per Brighton level 1 criteria and their stool samples were collected. Out of 17 cases, stool samples from four cases got contaminated and could not be used in further assays. Stool samples from eight controls (non-intussusception cases) were also collected for comparative analysis. The information regarding rotavirus vaccination was collected. Samples collected from cases and controls were transported safely to the referral laboratory once every month. Following inclusion criteria was considered in the current investigation.
Inclusion Criteria
Children were included as cases of intussusception if they meet the following inclusion criteria [8].
#Cases with <5 years of age who meet the case definition for intussusception using the Level 1 Brighton criteria [9].
#Surgical criteria:
Demonstration of invagination of the intestine at surgery;
#Radiological criteria:
Demonstration of invagination of the intestine by either air or liquid contrast enema;
Demonstration of an intra-abdominal mass by abdominal ultrasound with specific
characteristic features (target sign or doughnut sign on transverse section and a pseudo kidney or sandwich sign on longitudinal section), that is proven to be reduced by hydrostatic enema on post-reduction ultrasound.
#Autopsy criteria:
Demonstration of invagination of the intestine at autopsy.
Controls: Subjects selected from non-intussusception, non-infectious hospitalised patients were considered as controls in this study. Each control matched with a case in age, sex and location. Controls were taken within 30 days of the enrolment of intussusception cases. Written informed consent was obtained from the parent/primary caregiver.
Laboratory Procedures
Stool samples were stored at -70°C till further use. A screening for presence of rotavirus VP6 was performed by enzyme immunoassay or EIA (PremierTM Rotaclone, Meridian Bioscience Inc., Cincinnati, OH) [10]. The cDNA was used as template for genotyping in a hemi-nested multiplex PCR for detection of VP7 (G type) and VP4 (P type) genes, using published oligonucleotide primers [5,11]. For samples which were negative by genotyping PCR, a VP6 conventional PCR was performed to rule out rotavirus positivity [12].
Results
Prevalence of intussusception is presented in [Table/Fig-1], which shows that, during the study period, 17 infants and children with definite intussusceptions were identified, from a total of 2533 enrolled subjects aged <5 years (0.67%); whereas, 15 cases of the disease were recognised from a total of 1598 children aged <2 years of age (0.93%).
Prevalence of intussusceptions in hospital admitted children under 5 and 2 years of age.
| Total number of children admitted | Numbers of intussusception cases | Prevalence |
|---|
| <5 years-n=2533 | 17 | 0.67% |
| <2 years-n=1598 | 15 | 0.93% |
n=Numbers
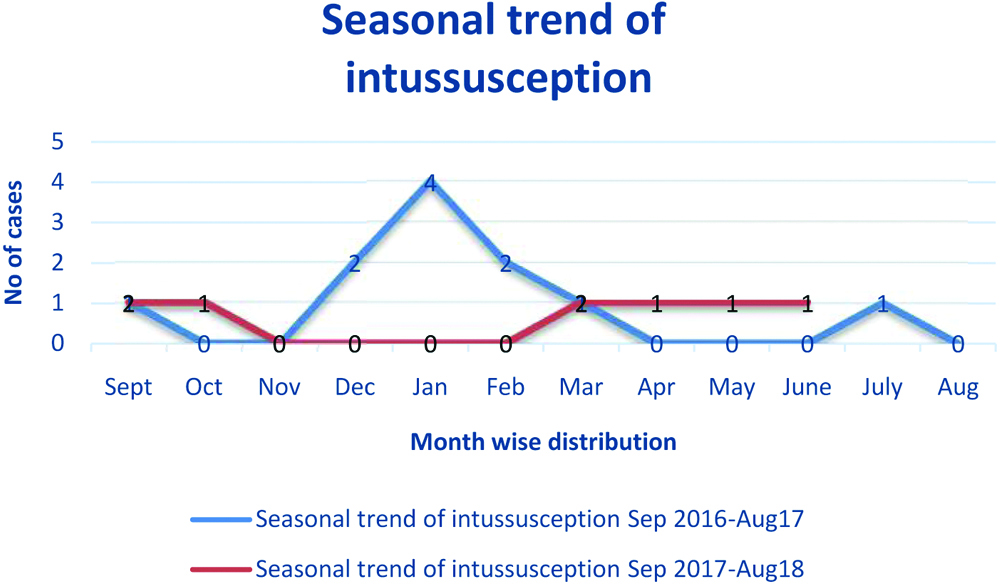
A line graph is shown to examine the seasonal trend in intussusception cases from Sept. 2016-Aug 2018 in KIMS, Odisha [Table/Fig-2]. It was observed that, intussusception cases occurred round the year with no distinct seasonality.
Seasonal trend of intussusceptions.
The majority (53%) of intussusception cases occurred in the second year of life [Table/Fig-3]. Further, the male: female ratio was found as 2.4:1. The prevalence of intussusception was higher in patients of rural areas (76%) as compared to urban areas (24%). Although fever, bloody stool and pain abdomen was the presentation in most of the cases (59%, 47%, 47% respectively), vomiting was found in 35% of cases. Constipation was present in 1 (6%) case. Lead point was identified in 7 (41%) cases. Intussusception was reduced by medical management in 15 (88%) of cases and 2 (12%) cases needed surgical intervention. Out of total 21 stool samples no rotavirus was detected in cases but one control sample (12.5%) was found positive [Table/Fig-3].
Socio-demographic profile and clinical features of 17 intussusceptions cases.
| Variables | Total number | (%) |
|---|
| Gender |
| Male | 12 | 70.5 |
| Female | 5 | 29.5 |
| Age |
| <12 months | 8 | 47 |
| >12 months | 9 | 53 |
| Location |
| Rural | 13 | 76 |
| Urban | 4 | 24 |
| Fever | 10/17 | 59 |
| Vomiting | 6/17 | 35 |
| Constipation | 1/17 | 6 |
| Bloody stools | 8/17 | 47 |
| Abdominal pain | 8/17 | 47 |
| Rotavirus detected in stool sample. |
| *Cases, n=13 | 0 | 0 |
| Control, n=8 | 1 | 12.5 |
| Management | | |
| Surgical | 2/17 | 12 |
| Medical | 15/17 | 88 |
*Stool samples from 13 cases were used out of total 17 for rotavirus detection. Eight controls (non-intussusception cases) were taken for comparative analysis.
As shown in [Table/Fig-4], the average age of onset of the disease was 15.47±13.14 months for cases aged <5 years; whereas, for subgroup with age <2 years, it was found to be 11.33+7.12 months. Next, the duration of hospital stay was 6.35±3.57 days and 4.37±0.91 days for cases and controls respectively. However, no intussusception associated death was recorded.
Age of onset and duration of hospital stay of intussusceptions cases.
| Characteristics | Control (n=8) (Mean±SD) | Case (Mean±SD) | p-value |
|---|
| Age of onset <5 years (in months) (n=17) | 17.38±13.82 | 15.47±13.14 | 0.75 |
| Age of onset <2 years (in months) (n=15) | 17.38±13.82 | 11.33+7.12 | 0.176 |
| Hospital stay (in days) | 4.37±.91 | 6.35±3.57* | 0.14 |
For cases (n=17)
As shown in [Table/Fig-5], on categorical characterisation of cases and controls, it was found that, fever and vomiting occurs in both groups showing no significant difference. However, bloody stool and pain abdomen were consistently found in cases, and not in controls (p<0.019). Importantly, rotavirus was detected in only one control stool sample.
Comparison of categorical characteristics between case and control.
| Character | Control (n%) | Case (n%) | p-value |
|---|
| Gender |
| Female | 5 (50) | 5 (50) | 0.194 |
| Male | 3 (20) | 12 (80) |
| Locality |
| Rural | 4 (24) | 13 (76) | |
| Urban | 4 (50) | 4 (50) | 0.359 |
| Fever | 7 (42) | 10 (58) | 0.205 |
| Vomiting | 4 (40) | 6 (60) | 0.667 |
| Constipation | 0 (00) | 1 (100) | 1.000 |
| Bloody stools | 0 (00) | 8 (100) | 0.019 |
| Abdominal pain | 0 (00) | 8 (100) | 0.019 |
| Rotavirus detected | 1 (100) | 0 (00) | 0.137 |
Discussion
WHO recommends surveillance of intussusception in countries where rotavirus vaccine programme is carried out. Although, prior studies have indicated no alterations in risk of intussusception during Rotavac® administration [13], we performed the current investigation to examine a baseline informations in our locality. The purpose of the present study was to monitor intussuception after rotavirus vaccine introduction in national immunization schedule.
We analysed intussusception data obtained in 2 years of study in Odisha. Being a referral hospital, the records for denominators of population and birth were not available to estimate the population based incidence of intussusceptions. However, the hospital based incidence of 6.7 out of 1000 admitted cases was observed in our study and the frequency was found to be higher as compared to other studies [14-16]. The incidence of intussusception in European children varied from 0.66 to 2.24 per 1000 admitted subjects and 0.75 to 1.00 per 1000 children in emergency departments. Although, the peak incidence of this disease was found in children between 3 to 9 months of age [17], the age range was found to be 11.33+7.12 months as shown by us. Similar to our findings, the median age was 12.1 months as observed in a study conducted at Uzbekistan [18].
In this study, the classic triad; including bloody stool, abdominal pain and fever were found in about 50% of cases; similar to reports made by other studies [19,20]. Fever was the most common clinical symptom and its absence makes the diagnosis of intussusception unlikely. The median length of hospital stay for patients in this study was 6.35+3.57 days, which is similar to other studies conducted in Uzbekistan [18] and Tanzania [21].
Although, available evidences to date do not indicate the risk of intussusception after vaccination, a vigilance surveillance is recommended to rule out such risk. The ability to access the risk of a rare adverse event will depend on the background rates of the event in the people who underwent vaccination. Present study, the first of its kind, aims to document the hospital based incidence and epidemiology of childhood intussusception in post vaccination programme Government of India. In this study, we observed the intussusception cases were not associated with rotavirus vaccination or natural rotavirus infection. This result is in accordance with earlier studies that indicated no increase in intussusception rate from baseline after rotavirus vaccination [22,23].
In majority of cases, the intensity of the disease was reduced due to early presentation to hospital and medical management, including immediate intervention by paediatric surgeon with help of radiologist [4,24]. In our case, only two cases needed surgical intervention. Additionally, no case fatality was observed which is consistent with another study [25].
Limitation
The major limitation of the study is that, being a tertiary care hospital, intussusception rate could not be calculated. Although intussusception is more prevalent in children aged <2 years, we considered the cases aged under 5 years, to see the epidemiological trend. Other infectious gut pathogens responsible for intussusception were not evaluated. Lastly, the numbers of cases and controls were also less to generalise.
Conclusion
In this preliminary investigation, neither rotavirus infection nor rotavirus vaccination in children <5 years of age attributed to intussusceptions. Overall, the study provides an information on a baseline epidemiology of childhood intussusception in children of Odisha. However, to examine the safety concerns about rotavirus immunization program, multicentric studies are crucial to perform and our future studies are directed to achieve this target.
Disclaimer: The findings and conclusions in this article are those of the author(s) and do not represents the view of CMC Vellore.
Funding: This study was funded by CMC Vellore.
Ethics approval and consent to participate: All infants’ parents provided written informed consent, and this study was approved by the Ethical Committee of Kalinga Institute of Medical Sciences, KIIT University and the Institutional Review Board of CMC, Vellore.
n=Numbers
*Stool samples from 13 cases were used out of total 17 for rotavirus detection. Eight controls (non-intussusception cases) were taken for comparative analysis.
For cases (n=17)