Cervical cancer is the second leading malignancy in women worldwide as approximately 510,000 new cases of cervical cancer is diagnosed annually with approximately 288,000 deaths worldwide [1]. More than 1,22,844 women in India are diagnosed with cervical cancer and more than 67,477 die of the disease every year [2]. It is expected that in India, cervical cancer occurs in around 1 in 53 women contrast with 1 in 100 women in more developed regions of the world [3]. Age, multiple pregnancies, number of abortions, use of oral contraceptives play an important role in development of cervical cancer. The relationship of Human Papillomavirus (HPV) and cervical cancer is well studied and the association of HPV in cervical cancer is as high as association of smoking in lung cancer [4].
HPV is very common worldwide and primarily transmitted through sexual contact in both males and females [5]. Approximately, 90% of HPV infections are asymptomatic and cleared by immune system. Epidemiological data and molecular observations have revealed that a persistent infection with high-risk HPV is the most crucial risk factor for cervical, anogenital and other organ sites cancer [6]. There exist more than 100 types of HPVs out of which more than 40 HPV types are found in the genital tract that is passed through sexual contact. Ten HPV types i.e., 16, 18, 31, 33, 35, 45, 51, 52, 58 and 59 have been adequately evaluated as high-risk types or oncogenic types [7]. Previous studies have strongly supported HPV infection as the prime risk factor in cervical cancer [8-12]. Being important risk factor, HPV needs to be studied in every part of the country as the incidence of HPV and its subtypes are different in different geographical regions of the country. Association of HPV with various risk factors like age, age at marriage, parity, residential background, menstrual status and menstrual hygiene also needs to be studied in cervical cancer for designing future screening and vaccination programs.
After considering all facts, the present study was done for the first time to account the prevalence of HPV infection in Haryana, India and aims to determine the incidence of high risk HPV types 16 and 18 in cervical cancer patients by using highly sensitive Polymerase Chain Reaction (PCR) technique. An effort was also made to find out association of HPV infection with socio-demographic factors and with histopathological factors in cervical cancers.
Materials and Methods
The present retrospective study was conducted on cervical tissue biopsies collected from 110 patients with cervical abnormalities (94 cases of cervical cancer and 16 cases of chronic cervicitis) of different age group from Department of Obstetrics and Gynaecology, Pandit Bhagwat Dayal Sharma Health University, Rohtak, Haryana, India, from September 2016 to August 2018. Married women were enrolled in the study after clinical examination. Relevant information regarding age, age at marriage, parity, number of abortions, menstrual hygiene, menstrual status, chief complaints, clinical diagnosis, examination findings, etc. were recorded.
Biopsy samples were histopathologically confirmed by histopathologists as cervicitis, squamous cell carcinoma, adenocarcinoma, adenosquamous carcinoma. Written informed consent was obtained from all participating patients. Processing of all samples was done in a laminar flow cabinet in Department of Genetics, Maharishi Dayanand University, Rohtak, Haryana, India. Ethical approval for sample collection was taken from institutional human ethical committee (IHEC) with Number IHEC/2016/80-13.06.16.
Sample collection and genomic DNA isolation: Cervical biopsies were collected and stored immediately at -80°C for further process. Briefly, tissues were crushed in Liquid N2 and resuspended in STE buffer (100 mM NaCl, 10 mM Tris and 1 mM EDTA) and incubated with 100 μg of proteinase K at 55°C for 16 hour. DNA was extracted by using phenol, chloroform and isoamyl alcohol mixture (25:24:1) and further precipitated by using ethanol [13].
DNA concentration and purity assay: The purity and concentration of genomic DNA was checked by Nanodrop spectrophotometer at OD 260/280. Integrity of genomic DNA was checked on 1% agarose gel. Quality of DNA samples was tested by GAPDH, yielding a human GAPDH product of 200 bp.
HPV detection and HPV typing: Following primers were used for the PCR amplification: For GAPDH, F 5’-AGCGAGATCCCTCCAAA- 3’ and R 5’-CTTGAGGCTGTTGTCATACT-3’; for GP5+/6+, F 5’-TTT GTT ACT GTG GTA GAT ACT AC-3’ and R 5’ GAAAAATAAA CT GTAAATCAT ATTC; for HPV 16, F 5’-ATT AGT GAG TAT AGA CAT TA-3’ and R 5’-GGC TTT TGA CAG TTA ATA CA-3’; for HPV 18, F 5’-CAC TTC ACT GCA AGA CAT AGA-3’and R 5’-GTT GTG AAA TCG TCG TTTTTC A-3’. HPV genotyping was done by using degenerate primers GP+5/+6 targeting 150 bp region and type specific PCR using HPV 16 and 18 specific primers targeting 109 and 334 bp region respectively. Reactions were performed as initial denaturation at 94°C for 4 minutes followed by 35 cycles with denaturation for 45 seconds at 94°C, annealing at 53-61°C for 45 seconds and extension at 72°C for 1 minute, and a final extension at 72°C for 7 minutes. PCR products were further visualised on ethidium bromide stained 2% agarose gel.
Statistical Analysis
Association of various risk factors and histopathological factors with the presence of HPV 16 and HPV 18 infections was estimated by using multivariate logistic regression analysis. Descriptive values were presented as mean, median, Standard Deviation (SD), percentages and frequencies. Statistical analyses were performed by using Medicalc version 18.9. Odd ratio (95% confidence interval) was calculated to check the association of HPV type 16 and 18 infections with sociodemographic factors and histological grades. A p-value of ≤0.05 was considered statistically significant.
Results
Sociodemographic and histopathological parameters for enrolled cases (110) were recorded. The association of HPV 16 and 18 infections with different parameters were studied.
Demographic and clinical features of cervical cancer cases: Sociodemographic features and histological grading of all cervical cancer cases are provided in [Table/Fig-1,2]. Mean age and age at marriage of the registered cases were 55.3 and 15.95 with an age range of 38-82 years and 14-21 years respectively. Most of the cases (84.39%) were illiterate/just literate and belonged to rural background (78.18%). Bleeding after menopause and bleeding during intercourse were the main symptoms in cervical cancer cases. Differentiation degree was unknown for 46 (41.81%) biopsies, 30 (27.27%) biopsies showed moderate differentiation, 10 (9.09%) showed well differentiation and 8 (7.27%) showed poor differentiation.
Sociodemographic factors as risk factors for HPV 16 and 18 infection in the study population of cervical cancer.
| Sociodemographic Factors | Total | HPV | HPV | OR (95%CI) | P | HPV | HPV | OR (95%CI) | P |
|---|
| N=110 | % | 16+ | 16- | 18+ | 18- |
|---|
| Age |
| ≤55 years | 42 | 38.18 | 33 | 9 | 0.7 (0.2-1.8) | 0.49 | 35 | 7 | 2.9 (1.1-7.5) | 0.027* |
| >55 years | 68 | 61.82 | 57 | 11 | 43 | 25 |
| Age at time of marriage |
| ≤18 | 53 | 48.18 | 47 | 6 | 2.55 (0.9-7.2) | 0.07 | 30 | 23 | 0.2 (0.1-0.6) | 0.002* |
| >18 | 57 | 51.81 | 43 | 14 | 48 | 9 |
| Rural | 86 | 78.18 | 78 | 8 | 9.7 (3.3-28.7) | 0.0001* | 60 | 26 | 0.8 (0.3-2.2) | 0.62 |
| Urban | 24 | 21.81 | 12 | 12 | 18 | 6 |
| Number of births |
| ≥3 | 60 | 54.54 | 52 | 8 | 2.0 (0.7-5.5) | 0.15 | 45 | 15 | 1.6 (0.7-3.5) | 0.30 |
| <3 | 50 | 45.45 | 38 | 12 | 33 | 17 |
| Number of abortions |
| 0-3 | 101 | 91.81 | 84 | 17 | 2.4 (0.5-10.8) | 0.23 | 72 | 29 | 1.2 (0.3-5.3) | 0.77 |
| ≤3 | 9 | 8.18 | 6 | 3 | 6 | 3 |
| Menopause |
| Pre | 43 | 39.09 | 31 | 12 | 0.4 (0.2-1.1) | 0.07 | 28 | 15 | 0.3 (0.1-0.74) | 0.009* |
| Post | 67 | 60.90 | 56 | 9 | 58 | 9 |
| Menstrual Hygiene |
| Poor | 72 | 65.45 | 50 | 22 | 1.1 (0.5-2.5) | 0.91 | 52 | 20 | 3.21 (1.4-7.3) | 0.005* |
| Good | 38 | 34.54 | 26 | 12 | 17 | 21 |
OR: Odd ratio; CI: Confidence of interval; No: Number; HPV: Human papillomavirus; P: p-value. *Significant at p≤0.05
Association of HPV 16 and 18 infections with histopathological factors.
| HPV Type infection | HPV infected (107) | Cervicitis | Squamous carcinoma | Adenocarcinoma | Adenosquamous |
|---|
| N (%) | OR (95% CI) | P | N (%) | OR (95% CI) | P | N (%) | OR (95% CI) | P | N (%) | OR (95% CI) | P |
|---|
| HPV16+ | 90 | 6 (37.5) | 0.133 (0.04-0.40) | 0.0004* | 81 (90) | 2.0 (0.86-4.64) | 0.10 | 1 (50) | 0.22 (0.01-3.70) | 0.29 | 2 (100) | 1.13 (0.05-24.4) | 0.93 |
| HPV16- | 20 | 10 (62.5) | 9 (10) | 1 (50) | 0 |
| HPV18+ | 78 | 3 (18.7) | 0.09 (0.025-0.35) | 0.0005* | 73 (82.2) | 1.76 (0.90-3.44) | 0.097 | 0 | 0.88 (0.03-1.77) | 0.11 | 2 (100) | 2.07 (0.09-44.31) | 0.64 |
| HPV18- | 32 | 13 (81.2) | 17 (18.88) | 2 | 0 |
OR: Odd ratio; CI: Confidence of interval; No: Number; HPV: Human papillomavirus; P: p-value. *Significant at p≤0.05
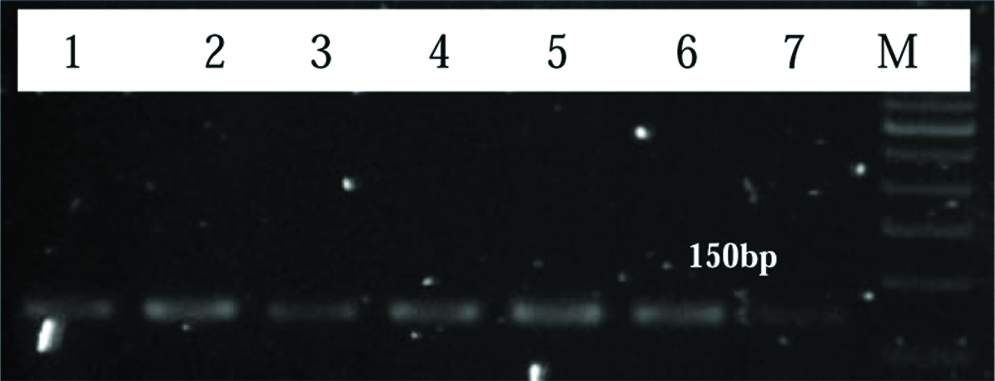
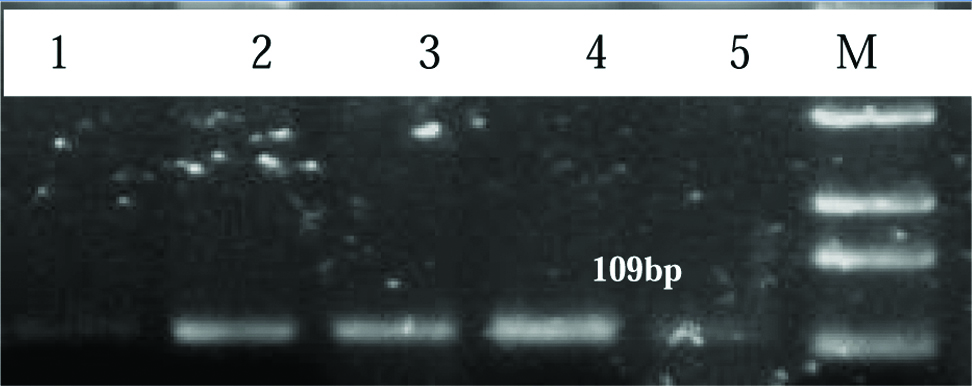
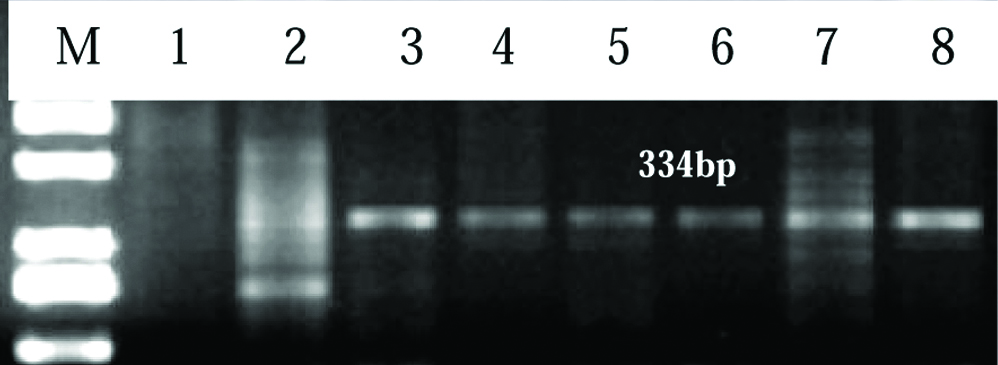
Detection of HPV by PCR in cervical cancer samples: Out of 110 cervical cancer samples, 107 (97.27%) were recorded positive for HPV DNA. Out of 107 HPV positive cervical cancer samples, 90 (84.11%) samples were found HPV 16 positive and 78 (72.89%) samples were found positive for HPV 18. These results suggest that the prevalence of HPV infection in cervical cancer is very high and HPV 16 is more prevalent along with HPV 18. [Table/Fig-3] represents 2% agarose gel showing 150 bp product for the GP05+/6+ consensus primers of HPV in cervical cancer patients. Similarly, [Table/Fig-4,5] represents 2% agarose gel showing 109bp and 334 bp product for HPV 16 and HPV 18 respectively.
Representative image of 2% agarose gel showing product of 150 bp targeting L1 gene (GP05+/6+ consensus primer) of HPV in cervical cancer patients. Lane M: Upto 1 kb DNA ladder, Lane 1 to 7 shows HPV positive samples.
Representative image of 2% agarose gel showing product of 109 bp for HPV 16 in cervical cancer patients. Lane M: Upto 1 kb DNA ladder, Lane 1 to 5 shows HPV positive samples.
Representative image of 2% agarose gel showing product of 334 bp for HPV 18 in cervical cancer patients. Lane M: Upto 1 kb DNA ladder, Lane 2-8 shows HPV positive samples and Lane 1 shows HPV negative.
Discussion
Cervical cancer is the most frequent malignancies in women worldwide accounting for 17% of all cancer deaths among women aged between 30 and 69 years. Although, the incidence of cervical cancer is steadily declining in the developed world; it is most common in developing countries [14]. In spite of being curable and preventable at an early stage, cervical cancer still causes more than 67,477 deaths annually in India due to the lack of organized screening programs and intervention approaches [2].
Although, India is a diverse country with extensive ethnicity, and socio-cultural diversity, the incidence of HPV infection may vary significantly in different regions so, it is required to study prevalence of HPV and its genotypes in every part of the country. The available literature shows that the prevalence of HPV in women in different parts of India ranges from 9 to 94%. Incidence of HPV 16 infection along with HPV 18 in cervical carcinoma is very high as compared to other HPV type infections in India [Table/Fig-6] [9-12,15-20].
Comparison of studies of HPV in cervical cancer from different regions of India [9-12,15-20].
| Sr No. | Place | Subjects enrolled | Number | Positivity | References |
|---|
| 1 | Chennai | HPV as a risk factor in cervical cancer patients | 205 | 99.4% (HPV DNA) | Franceschi S et al., [9] |
| 2 | Tamil Nadu | Women randomly selected from general population | 1179 | 14.2% (HPV DNA) | Clifford GM et al., [20] |
| 3 | Andhra Pradesh | HPV prevalence in cervical cancer and normal women | 41 | HPV DNA (87.8%)HPV 16 (66.7%)HPV 18 (19.4%) | Sowjanya AP et al., [10] |
| 4 | Chandigarh | Hospital based study of women with benign cervical cytology | 472 | 36.8% (HPV DNA) 8.2% (HPV 16 & 18) | Aggarwal R et al., [15] |
| 5 | North-Eastern India | HPV prevalence in women of eastern India without cervical cancer | 2501 | 9.9% (HPV DNA), 2% (HPV 16 & 18) | Datta P et al., [16] |
| 6 | Uttar Pradesh | A population based study of asymptomatic women of eastern Uttar Pradesh | 2424 | 9.9% (HPV DNA)s | Srivastava S et al., [17] |
| 7 | Amritsar | Prevalence of HPV infection in patients with cervical lesions | 100 | 10% (HPV DNA), 6% (HPV 18) and 2% (HPV 16) | Kaur P et al., [18] |
| 8 | Jammu and Kashmir | Hospital based study for HPV prevalence in women of Jammu Region | 500 | 40.8% (HPV DNA), 2% (HPV 16) and 6.8% (HPV18) | Bhardwaj R et al., [19] |
| 9 | Madhya Pradesh | HPV detection in cervical cancer patients | 52 | 84.6% (HPV DNA), 50% (HPV 16) and 15.3% (HPV18) | Bhandari V et al., [12] |
| 10 | Odisha | HPV Genotypes distribution in Indian women with and without cervical carcinoma | 607 | HPV DNA (93.28%) HPV 16 (87.28%), HPV18 (24.56%) | Senapati R et al., [11] |
In present study, the prevalence of HPV infection (97.27%) was found very high with HPV 16 (84.11%) and HPV 18 (72.89%) as the prominent infection. The prevalence of HPV types found in this study is quite similar to a recent large-scale study reported from Odisha, India [11].
Age at marriage and menstrual hygiene showed significant association with HPV 18 infection; moreover, a significant association between HPV 16 infection and residential background (p≤0.05) was also observed in this study group.
Present study proves that women above 55 year (CI=1.12-7.51 and p=0.027) and with early age at marriage (CI=0.1-0.6 and p=0.002) were at greater risk of developing HPV infection in cervical cancer cases i.e., 68 (61.82%) and 53 (48.18%) respectively which was quite similar to other studies [21-23]. So, it is believed that if females get vaccinated with HPV in younger age before their first sexual contact then, virus exposure can be stopped. The vaccine can put a stop to almost 100% of disease caused by the four types of HPV (HPV 16, HPV 18, HPV 6 and HPV11) [1].
People living in rural area are 20 times more prone to acquire HPV infection as these areas reflects poor socioeconomic condition due to lack of access to hospitals, poor genital hygiene, poor health [24]. In rural areas of Haryana, sexual notion and behavior of women are more conservative which stops women to talk about gynaecological problems. In present study, 86 (78.18%) of the cases were from rural background of Haryana with very high infection of HPV 16 (90.70%) and moderate with HPV18 (69.76%). It was observed that the women from rural areas of Haryana were at greater risk of developing HPV 16 infection than women living in urban areas of Haryana (CI=3.30-28.75, p=0.0001).
In villages, some women use old poorly stored clothes as pads and reuse them every time were considered as risk factors for infection. It was found that such women with poor menstrual hygiene (65.45%) were at greater risk of developing HPV infection (CI=1.4-7.3, p=0.004) than women with good menstrual hygiene (use sanitary pads). In these studies, no significant association was observed in post-menopausal state and HPV infection as compared to other significant study (p=0.009) [25].
The association of different histological groups of cervical cancer with HPV 16 and 18 infections was shown in [Table/Fig-2]. The risk of HPV 16 and 18 was significantly associated with cervicitis (p=0.0004 and 0.0005 respectively).
Due to high prevalence of HPV in Haryana region, it is extremely crucial to establish screening programs to make people aware about signs, symptoms, risk factors and preventive measures associated with cervical cancer.
Limitation
Small sample size and limited genotype (HPV 16 and 18) are major limitation for this study. Studies for larger population needs to be done to further validate these findings and provide strong evidence to make any further policies. Study of other high risk and low risk genotypes will help in knowing whether they contribute to the pathogenesis of cervical cancer.
Conclusion
Outcomes of the study suggest that the incidence of HPV in cervical cancer patients is high and strongly supports HPV as a causative factor in the development of cervical cancer. The infection of HPV 16 along with HPV 18 is found to be most prevalent in this study population. Thus, an effective vaccination program based on regional epidemiological profile of HPV is needed to reduce the cervical cancer burden in women of Haryana, India.
Funding: Work was supported by Medical Genetics laboratory, Department of Genetics, Maharishi Dayanand University, Rohtak, Haryana, India.
OR: Odd ratio; CI: Confidence of interval; No: Number; HPV: Human papillomavirus; P: p-value. *Significant at p≤0.05
OR: Odd ratio; CI: Confidence of interval; No: Number; HPV: Human papillomavirus; P: p-value. *Significant at p≤0.05