Diabetes mellitus, the most common endocrine disorder causes significant impairment of the various organ systems of the body including skin. The prevalence of dermatological disorders seen in diabetic patients is around 32% [1]. Prolonged hyperglycaemia results in production of advanced glycosylated end products which in turn is responsible for most of the complications of diabetes. The pathogenic mechanisms like abnormal carbohydrate metabolism, atherosclerosis, microangiopathy, neuron degeneration and impaired host mechanisms can be the causes of various dermatoses in diabetes [2].
Cutaneous manifestations of diabetes mellitus usually appear subsequent to the development of the disease, but sometimes it may be the first presenting sign and in some cases they may even precede the onset of primary disease by many years [3]. Therefore, early recognition of these manifestations will help to achieve good glycaemic control and prevent morbidities. Many similar studies had been conducted throughout the country [1,2]. Studies done by Raghu TY et al., and Rao GS et al., showed infections as the major cutaneous manifestations among diabetics and fungal infections accounted for the majority of infections [1,2], but only very few studies exist from Tamil Nadu and Pondicherry [3]. This made us to conduct this study. The aim of this work is to analyse the pattern of various cutaneous lesions among diabetic patients and to compare it with HbA1C levels.
Materials and Methods
A prospective case control study was conducted at RMMCH for a period of two years from November 2013 to October 2015. RMMCH is a teaching medical institution situated in a rural town catering to the needs of 4,69,416 population. A total number of 300 diabetic patients with various dermatoses were enrolled as study group. Sample size was not calculated. It was just fixed based on the previous studies [4,5]. Hundred non diabetic patients with various dermatoses, constituted the control group. All confirmed cases of diabetes with cutaneous manifestations irrespective of age, sex, associated diseases and those who were willing to participate in the study were included in the study group and all non diabetics with cutaneous manifestations were taken as control group.
Patients who did not give consent and gestational diabetes patients were excluded from the study. The ethical clearance was sought from the institutional ethical committee before starting the study. Informed consent has been obtained from all patients. All relevant details were entered in the case report form and associated comorbid conditions were also noted. All patients underwent a detailed dermatological examination. Various investigations like fasting blood sugar, post prandial blood sugar, glycosylated haemoglobin (HbA1C) levels, renal function test, lipid profile and urine analysis were done for all patients. Gram stain, KOH mounts and skin biopsy were done in relevant cases to confirm the dermatological diagnosis. The classification of cutaneous manifestations in diabetes proposed by Sreedevi C had been adapted for this study [Table/Fig-1] [6].
Classification of cutaneous manifestations in diabetes [6].
| Serial No | Classification | Dermatoses |
|---|
| 1 | Strongly associated but not specific for diabetes (disease markers) | Diabetic bullaeNecrobiosis lipoidica diabeticorumDiabetic dermopathyGranuloma annulareScleroderma like syndromePruritus |
| 2 | Due to diabetic complications | Diabetic footCutaneous infectionsXanthomatosisXanthelasmaPhycomycetesMalignant otitis media |
| 3 | Due to neurovascular complications | MicroangiopathyMacroangiopathyDiabetic neuropathy |
| 4 | Due to diabetes treatment | With Oral hypoglycaemic drugsWith Insulin |
| 5 | Endocrine syndromes with diabetes mellitus | Migratory necrolytic erythema |
| 6 | Commonly associated with diabetes mellitus | PsoriasisLichen planusVitiligoPerforating dermatosesEruptive xanthomasBullous pemphigoidDermatitis herpetiformisKaposi sarcoma |
Statistical Analysis
Descriptive statistical analysis was done using software SPSS version 21. Chi-square test was used for the comparison. Level of significance was fixed as 5% (p-value <0.05).
Results
The demographic details of the patients in both the groups are shown in [Table/Fig-2].
| Factor | Study group (N-300) | Control group (N-100) |
|---|
| Age | No | % | No | % |
|---|
| <20 yrs | 1 | 0.33 | 0 | 0 |
| 21-30 yrs | 4 | 1.33 | 5 | 5 |
| 31-40 yrs | 22 | 7.33 | 27 | 27 |
| 41-50 yrs | 84 | 28.00 | 27 | 27 |
| 51-60 yrs | 100 | 33.33 | 26 | 26 |
| 61-70 yrs | 57 | 19.00 | 7 | 7 |
| >70 yrs | 32 | 10.67 | 8 | 8 |
| Sex |
| Male | 175 | 58.3 | 53 | 53.0 |
| Female | 125 | 41.7 | 47 | 47.0 |
| Duration of Diabetes |
| <1 yrs | 70 | 23.3 | - | - |
| 1-5 yrs | 101 | 33.7 | - | - |
| 6-10 yrs | 52 | 17.3 | - | - |
| >10 yrs | 77 | 25.7 | - | - |
| Type of DM |
| Type I | 3 | 1.0 | - | - |
| Type II | 297 | 99.0 | - | - |
| Family History DM |
| No | 153 | 51.0 | 63 | 63.0 |
| Yes | 147 | 49.0 | 37 | 37.0 |
| Associated Co-morbidity |
| Absent | 124 | 41.3 | 80 | 80 |
| Present | 176 | 58.7 | 20 | 20 |
| Pattern Co-morbidity |
| Hypertension | 130 | 43.3 | 15 | 15.0 |
| Dyslipidemia | 83 | 27.7 | 5 | 5.0 |
| Coronary artery disease | 33 | 11.0 | 1 | 1.0 |
| Bronchial asthma | 16 | 5.3 | 1 | 1.0 |
| Hypothyroidism | 16 | 5.3 | 1 | 1.0 |
| Tuberculosis | 3 | 1.0 | 0 | - |
| Dermatoses |
| Infectious | 126 | 42 | 13 | 13.0 |
| Non infectious | 174 | 58 | 87 | 87.0 |
| No of Dermatoses |
| Single | 246 | 82.00 | 97 | 97.0 |
| Multiple | 54 | 18.00 | 3 | 3.0 |
| HBA1C |
| <6.4 | 59 | 19.7 | 100 | 100 |
| 6.5-7 | 70 | 23.3 | 0 | - |
| 7.1-8 | 103 | 34.3 | 0 | - |
| >8 | 68 | 22.7 | 0 | - |
[Table/Fig-3] showed the pattern of cutaneous manifestations of diabetic patients which revealed that infections were the commonest, as seen in 126 (42%) patients. [Table/Fig-4] showed the pattern of infections among study group and control group which revealed that infections were less in the control group. In [Table/Fig-5] the frequency of infections had been compared with HbA1C levels in the study group. It revealed that all the nine patients with multiple infections had uncontrolled diabetes.
Pattern of cutaneous manifestations in study group.
| Dermatoses | No. of patients | Percentage |
|---|
| Infections | 126 | 42.0% |
| Commonly associated dermatoses in diabetes mellitus | 103 | 34.3% |
| Less common dermatoses | 55 | 18.3% |
| Neuropathic and ischaemic | 27 | 9% |
| Most closely associated dermatoses in diabetes mellitus | 21 | 7% |
| Reaction to diabetes treatment | 1 | 0.3% |
Pattern of infections in study group and control group.
| Infections | Study group (N=300) | Control group (N=100) |
|---|
| Number of patients | Percentage | Number of patients | Percentage |
|---|
| Fungal alone | 87 | 29 | 8 | 8 |
| Bacterial alone | 29 | 9.7 | 5 | 5 |
| Viral | 8 | 2.6 | 0 | 0 |
| Fungal and bacterial | 2 | 0.7 | 0 | 0 |
In study group-Total no of patients with fungal infections: 89; Total no of patients with bacterial infections: 31; N=300 represents total number of patients in study group; N=100 represents total number of patients in control group
Comparison of number of infections in study group and HbA1C levels.
| No. of infections | HBA1C | Total |
|---|
| <7 | >7 |
|---|
| N | % | N | % | N | % |
|---|
| Single infection | 26.0 | 22.2 | 91.0 | 77.8 | 117.0 | 100.0 |
| Multiple infections | 0 | 0 | 9 | 100 | 9 | 100 |
[Table/Fig-6] compared the pattern of bacterial infections in study group patients and HbA1C levels. Furuncle was seen in 10 patients and out of which 9 patients had uncontrolled diabetes. Carbuncle was diagnosed in one patient with uncontrolled diabetes. [Table/Fig-7] revealed the pattern of fungal infections among study group compared with HbA1C levels. Interestingly a life threatening infection, mucor mycosis was diagnosed in two patients who had uncontrolled diabetes. [Table/Fig-8] showed the detailed pattern of number of infections among study group on comparison with HbA1C levels. All seven patients with two fungal infection had uncontrolled diabetes. [Table/Fig-9] compared the pattern of viral infections among study group with HbA1C levels. Out of eight patients with viral infections six patients had uncontrolled diabetes.
Pattern of bacterial infections in study group vs HbA1C.
| Bacterial infection | HBA1C | Total No-31 |
|---|
| <7 | >7 |
|---|
| No | % | No | % | No | % |
|---|
| Acute paronychia | 1 | 100.0 | 0 | 0.0 | 1 | 100 |
| Carbuncle | 0 | 0.0 | 1 | 100.0 | 1 | 100 |
| Cellulitis | 1 | 14.3 | 6 | 85.7 | 7 | 100 |
| Folliculitis | 1 | 33.3 | 2 | 66.7 | 3 | 100 |
| Furruncle | 1 | 10.0 | 9 | 90.0 | 10 | 100 |
| Hansens disease | 1 | 100.0 | 0 | 0.0 | 1 | 100 |
| Hidradenitis suppurativa | 1 | 100.0 | 0 | 0.0 | 1 | 100 |
| Infective eczematoid dermatitis | 0 | 0.0 | 1 | 100.0 | 1 | 100 |
| Pitted keratolysis | 0 | 0.0 | 1 | 100.0 | 1 | 100 |
| Pyoderma | 1 | 20.0 | 4 | 80.0 | 5 | 100 |
Comparison of fungal infections in study group Vs HbA1C value.
| Fungal infection | HBA1C | No of infections N=96 |
|---|
| <7 | >7 |
|---|
| N | % | N | % | N | % |
|---|
| Candidal balanoposthitis | 0 | 0 | 15 | 100 | 15 | 100 |
| Candidal intertrigo | 0 | 0.0 | 5 | 100.0 | 5 | 100 |
| Dermatophyte | 13 | 22.8 | 44 | 77.2 | 57 | 100 |
| Mucor mycosis | 0 | 0.0 | 2 | 100.0 | 2 | 100 |
| Oral candidiasis | 0 | 0.0 | 2 | 100.0 | 2 | 100 |
| Pityriasis versicolor | 4 | 28.6 | 10 | 71.4 | 14 | 100 |
| Vulvo vaginal candidiasis | 0 | 0.0 | 1 | 100.0 | 1 | 100 |
Out of 89 patients having fungal infections 7 patients had two fungal infections. So total number of fungal infections comes to 96
Comparison of number of infection in study group with HBA1C levels.
| Infections | HBA1C | Total |
|---|
| <7 | >7 |
|---|
| Number of patients | Number of patients | Number of patients |
|---|
| Single bacterial | 7 | 22 | 29 |
| Bacterial and fungal | 0 | 2 | 2 |
| Single fungal | 17 | 63 | 80 |
| Two fungal | 0 | 7 | 7 |
| Single viral | 2 | 6 | 8 |
Comparison of viral infections in study group Vs HbA1C levels.
| Viral infection | HBA1C | Total No: 8 |
|---|
| <7 | >7 |
|---|
| N | % | N | % | N | % |
|---|
| Herpes zoster | 1 | 25.0 | 3 | 75.0 | 4 | 100 |
| Warts | 1 | 25.0 | 3 | 75.0 | 4 | 100 |
Pattern of dermatoses commonly associated with diabetes had been depicted in [Table/Fig-10]. Less common dermatoses were represented in [Table/Fig-11]. Pattern of the neuropathic and ischemic skin diseases is depicted in [Table/Fig-12].
Pattern of dermatoses commonly associated with diabetes.
| Dermatoses | No of patients |
|---|
| Acquired perforating disorders | 4 |
| Amyloidosis | 8 |
| Bullous pemphigoid | 3 |
| Eruptive xanthoma | 1 |
| Lichen planus | 11 |
| Psoriasis | 45 |
| SLE | 1 |
| Urticaria | 25 |
| Vitiligo | 7 |
| Xanthelasma | 1 |
When the same patient had two dermatoses under the same group, that patient had been counted for both of those dermatoses. This is the reason for the discrepancy in numbers between this table and [Table/Fig-3].
Pattern of less common dermatoses in study group.
| Dermatoses | No of patients N=55 |
|---|
| Bullous leucocytoclastic vasculitis | 1 |
| Contact dermatitis | 10 |
| Eczemas | 14 |
| Leiomyoma cutis | 2 |
| Polymorphic light eruption | 5 |
| Scabies | 7 |
| Others | 16 |
16 patients had other dermatoses like keloid (4), melasma (3), erythroderma (3), alopecia areata (3) and androgenetic alopecia (3)
Pattern of Neuropathic and ischemic skin diseases in study group.
| Dermatoses | No of patients |
|---|
| Asteatotic eczema | 11 |
| Diabetic foot ulcers | 11 |
| Fissure feet | 1 |
| Notalgia paresthetica | 1 |
| Peripheral neuropathy | 5 |
When the same patient had two dermatoses under the same group, that patient had been counted for both the dermatoses
The pattern of most closely associated dermatoses had been shown in [Table/Fig-13]. The patient who presented with diabetic bulla had uncontrolled diabetes i.e., HbA1C >7.
Pattern of most closely associated dermatoses in study group.
| Dermatoses | No of patients |
|---|
| Acanthosis nigricans | 2 |
| Acquired icthyosis | 3 |
| Diabetic bulla | 1 |
| Diabetic hand syndrome | 2 |
| Granuloma annulare | 2 |
| Pruritus | 11 |
| Skin tags | 4 |
When the same patient had two dermatoses under the same group, that patient had been counted under both the dermatoses
[Table/Fig-14] analysed the relation between cutaneous manifestations and control of diabetes. In this study group of 300 patients, we noticed 333 cutaneous manifestations and of which 198 manifestations occurred in uncontrolled diabetes i.e., HbA1C >7. Among diabetics out of all the groups, only infections had statistically significant association with control of diabetes i.e., p-value <0.05 which means infections were more common in uncontrolled diabetes (HbA1C >7).
Comparison of cutaneous manifestations among study group in relation to control of diabetes.
| Group | HBA1C | Total | p-value |
|---|
| <7 | >7 |
|---|
| Most closely associated dermatoses | 11 | 10 | 21 | 0.368 |
| Infections | 26 | 100 | 126 | 0.021 |
| Neuropathic and ischaemic | 13 | 14 | 27 | 0.571 |
| Reaction to diabetes treatment | 0 | 1 | 1 | 0.384 |
| Commonly associated dermatoses in DM | 47 | 56 | 103 | 0.506 |
| Less common dermatoses | 38 | 17 | 55 | 0.029 |
| Total | 135 | 198 | 333 | |
Chi-square test was used for the comparison to calculate p-value. Level of significance was fixed as 5% (p-value <0.05)
Clinical pictures of few dermatoses like dermatophytosis, candidal balanoposthitis, carbuncle, mucor mycosis, diabetic bulla, eruptive xanthoma and insulin lipodystrophy were shown in [Table/Fig-15,16,17,18,19,20 and 21] respectively.
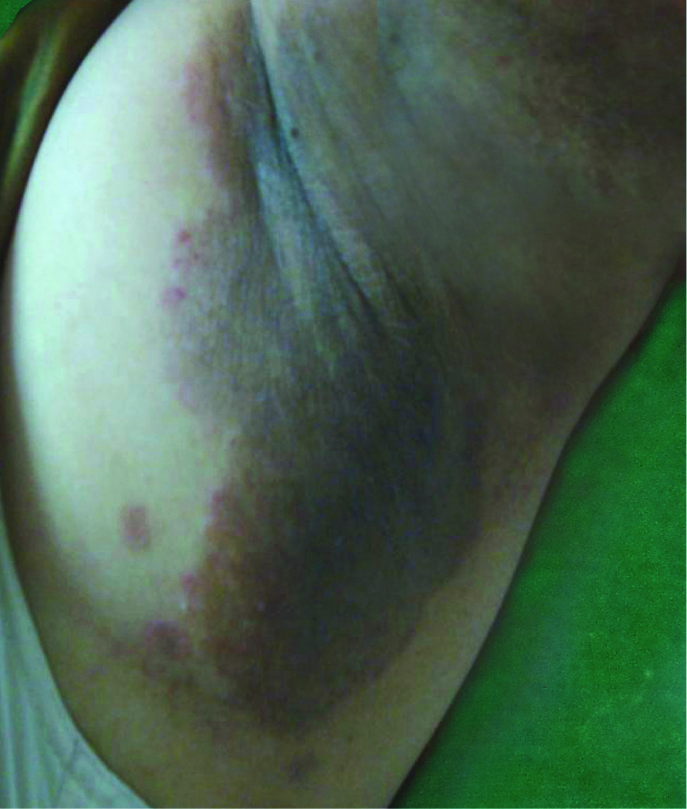
Dermatophytosis. Well defined hyperpigmented patch with central clearing and erythematous papules in the margins in axilla suggestive of dermatophytosis.
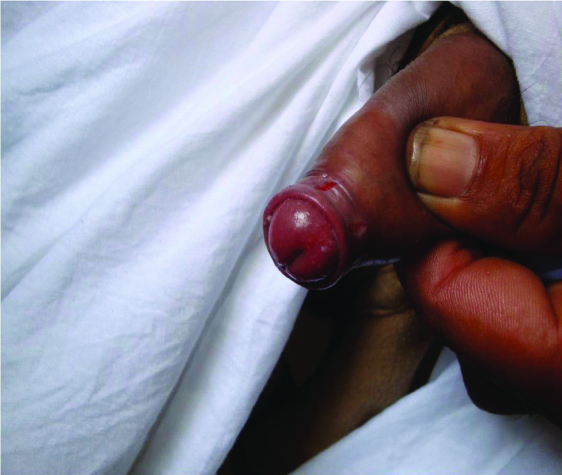
Candidal balanoposthitis. Multiple fissures over the prepuce suggestive of candidal balanoposthitis.
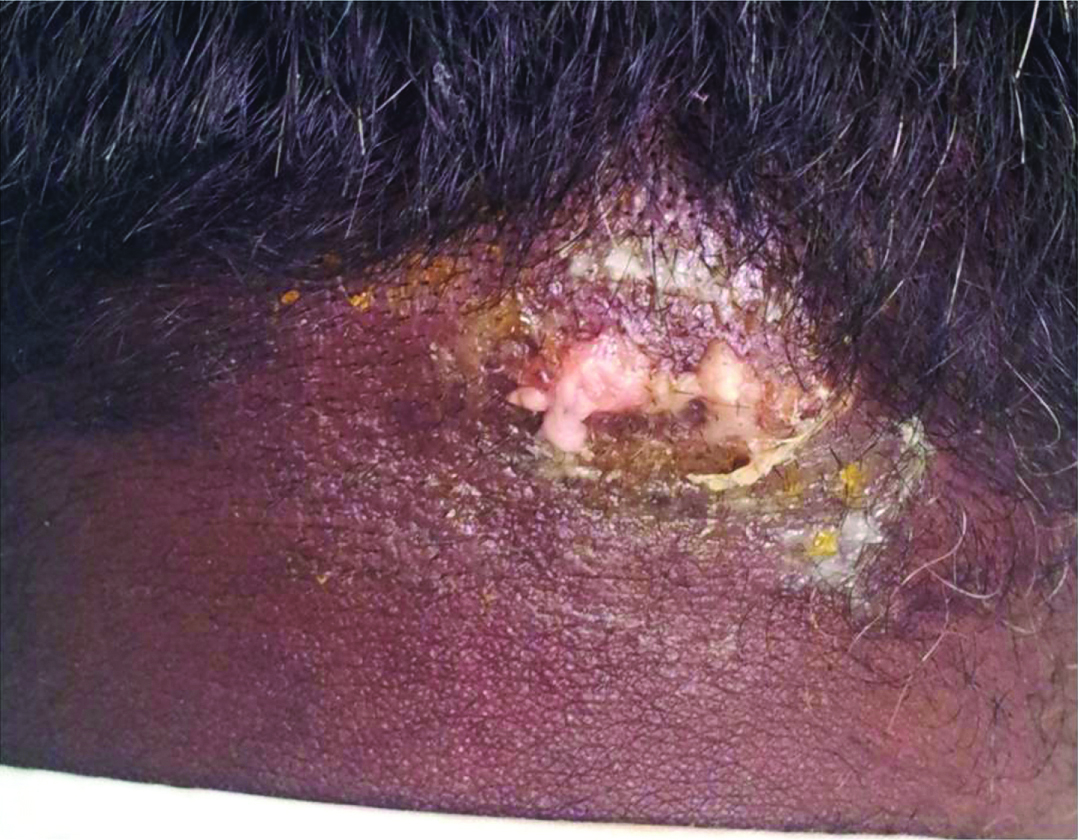
Carbuncle. Carbuncle over the nape of the neck.
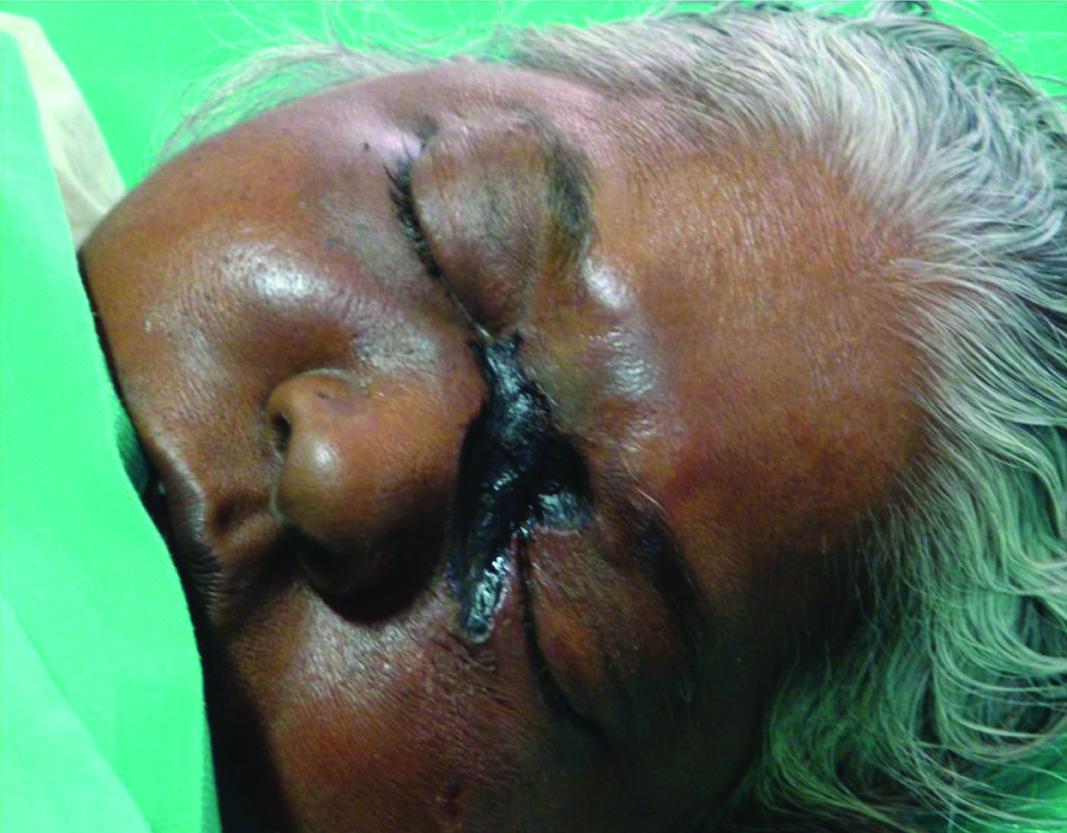
Mucor mycosis. Well defined necrotic plaque with blackish eschar seen over medial canthi, bridge of the nose and left infra orbital region in mucor mycosis.
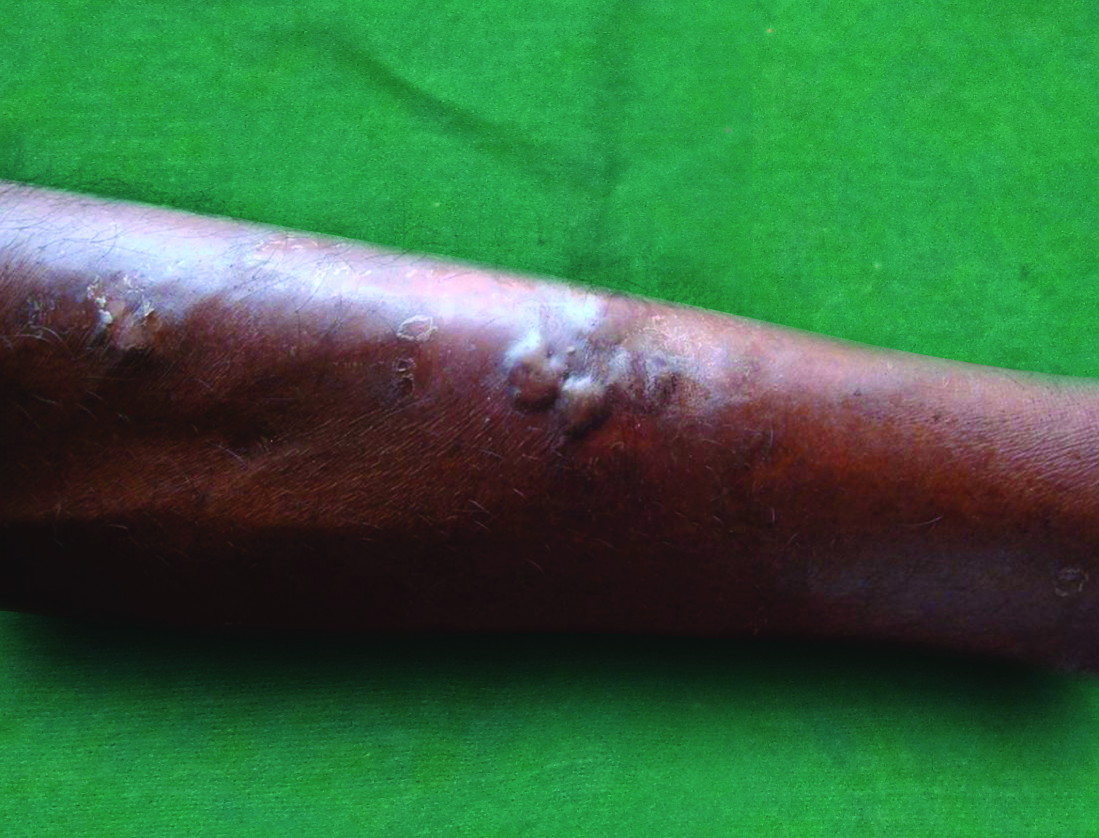
Diabetic bulla. Multiple tense bulla present over the shin suggestive of diabetic bulla.
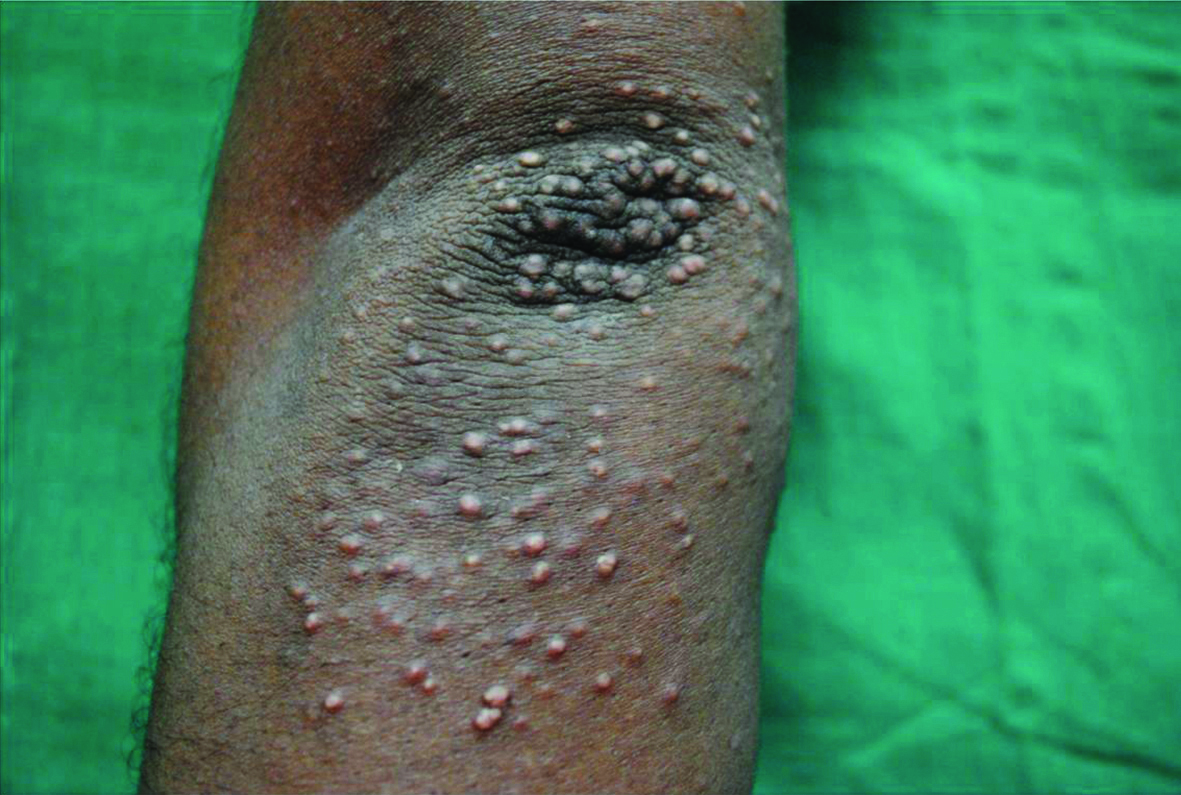
Eruptive Xanthoma. Multiple skin coloured to yellowish papules over extensor aspect of elbow suggestive of eruptive xanthoma.
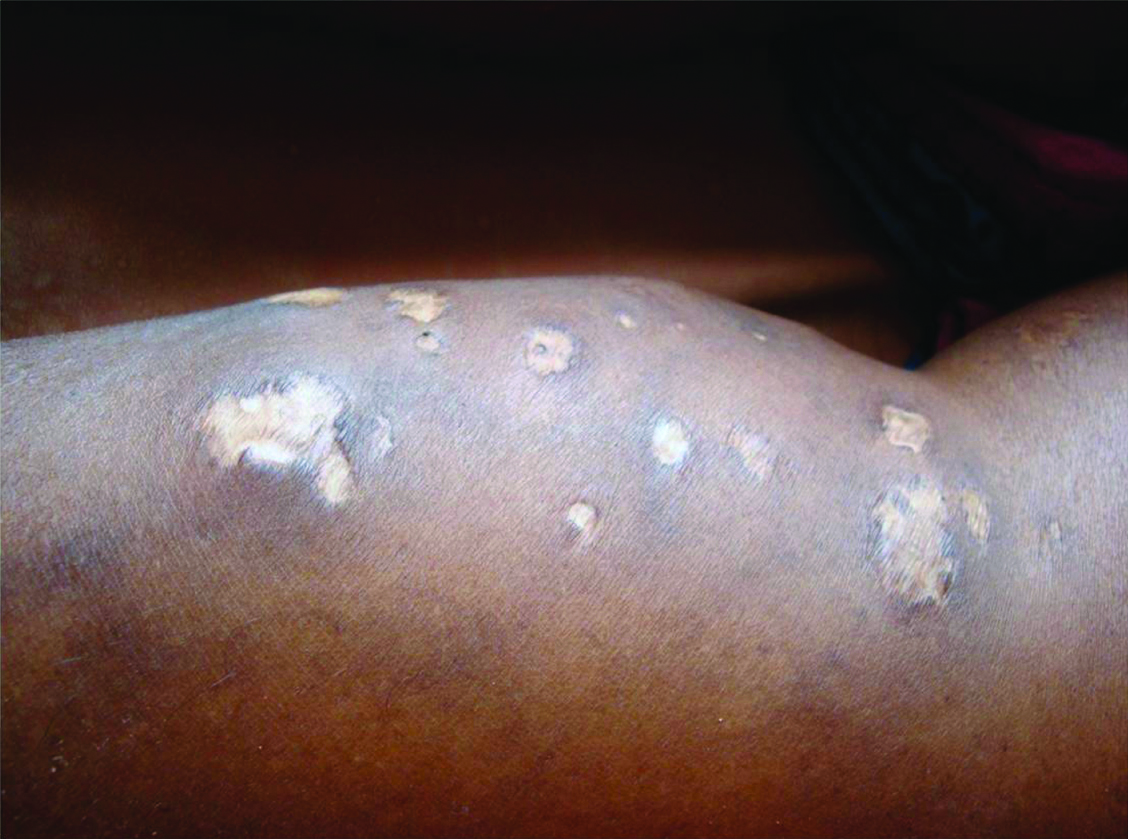
Insulin lipodystrpohy. Multiple atrophic plaques present at the sites of insulin injection suggestive of insulin lipo dystrophy.
Discussion
Diabetes-associated skin conditions can be a direct result of the metabolic changes such as hyperglycaemia, or to progressive damage to the vascular, neurological or immune system [3]. Age group in diabetic patients correlated with a study conducted by Timshina DK et al., from a neibhouring town [3]. In contrast Bhat YJ et al., reported the common age group as 41-50 years [7]. Positive family history was noticed in more number of patients when compared to that of control group. This signifies the importance of regular screening for diabetes among patients with family history of diabetes. Associated co-morbidities were seen in more number of diabetic patients when compared to that of control group. Study done by Bhat YJ et al., showed similar pattern of co-morbidity like this study whereas study by Raghunatha S et al., revealed a lesser incidence of co-morbid conditions which could have been due to difference in population [7,8]. Hypertension was the commonest co-morbid condition associated with this study group patients. The relationship between hypertension and diabetes was explained by Mahajan S et al., where he hypothesized that hypertension was found to accelerate the process of micro-angiopathy among diabetics [9]. In this study we observed that 57% of the patients had uncontrolled diabetes. This could be due to the lack of awareness about the disease and its complications which could be attributed to lack of primary level education in 58% of patients. In contrast to this study, Rajendra KJ et al., and Mashkoor AW et al., observed less number of patients with uncontrolled diabetes [10,11]. The diabetic patients were selected from medicine and endocrinology departments respectively for these studies. Hence, all the patients were given adequate counseling regarding the importance of glycaemic control and all were under regular follow-up, which could be the reason for less number of patients with uncontrolled diabetes in these studies.
Infections were the commonest dermatoses among the study population which had a statistically significant association with the glycosylated haemoglobin levels. That means HbA1C levels (HbA1C >7) had a direct correlation to the incidence of infections whereas no significant association between glucose levels and occurrence of skin manifestations was observed in studies conducted by Rajendra KJ et al., Nandhini C et al., and Fady SY et al., [10,12,13]. Similarly on comparing the frequency of infections with HbA1C levels showed that all the patients with multiple infections had uncontrolled diabetes. Studies done by Vahora R et al., Mahajan S et al., and Al-Mutairi N et al., also showed infections as the major manifestation, similar to this study [4,9,14]. Raghu TY et al., Timshina DK et al., Bhat YJ et al., Raghunatha S et al., Al-Mutairi N et al., and Abhishek G et al., showed that fungal infections were the commonest which was similar to this study [1,3,7,8,14,15]. Khurshid A et al., and Rajendra KJ et al., reported bacterial infections more commonly than fungal infections, which were seen in 160(64.5%) and in 67 (19%) patients [5,10]. The causes of infections in diabetes were due to the following factors: a) Hyperosmolality of the hyperglycaemic serum which causes diminished chemotaxis; b) Impaired release of cytokines as a consequence of lack of insulin; c) Impaired phagocytosis, which may be due to diminished leukocyte response and associated neuropathy [1]. Infections were mostly prevalent during early diabetes because the decrease in the host defense mechanism and impaired phagocytosis were noticed immediately in diabetics and these changes do not require much longer time to develop unlike microangiopathy [2].
More number of patients were observed with psoriasis, urticaria, lichen planus, cutaneous amyloidosis, vitiligo acquired perforating disorders, bullous pemphigoid and xanthelasma. There was one patient with eruptive xanthoma with hypertriglyceridemia who was diagnosed to have diabetes based only on clinical presentation. Psoriasis had been significantly associated with diabetes [4,16,17]. An association between psoriasis and increased cardiovascular risk and metabolic syndrome has been reported. Hence, while treating a patient with psoriasis, screening for metabolic syndrome and cardiovascular risk factors are advised [4]. Vitiligo can occur in diabetes as a part of multiple auto immune dysfunction. Bhat YJ et al., Raghunatha S et al., Mahajan S et al., Al-Mutairi N et al., Neerja P, Mahmood F et al., observed lesser number of patients under the category of dermatoses commonly associated with diabetes [7-9,14,18,19]. Among 27 patients with neuropathic and ischaemic dermatoses, diabetic foot ulcers and asteatotic eczema were more common in uncontrolled diabetes but the association was not statistically significant. Girisha BS observed 12 patients having diabetic foot ulcer similar to this study [20]. Comparison of important dermatoses in this study with other studies has been shown in [Table/Fig-22] [2,6,8,11,13,15,18,20-22]. Only 21 patients had dermatoses closely associated with diabetes. Only one case of bullous diabeticorum this osbserved. Studies done by Rao GS et al., and Girisha BS et al., showed similar number of patients having diabetic bulla, seen in two and one patient respectively [2,20]. Again these manifestations also were not significantly associated with blood sugar control. One patient had insulin lipodystrophy in this study. Use of human insulin may be the reason for absence of insulin reaction in this study [8].
Comparison of the important dermatoses in present study with other studies [2,6,8,11,13,15,18,20-22].
| Dermatoses | Present study | Studies with similar frequencies | Studies with different frequencies |
|---|
| Fungal infections | 89 | 106 [20]69 [6] | 24 [18]31 [15] |
| Bacterial infections | 31 | 20 [18]34 [6] | 67 [15]80 [11] |
| Diabetic bulla | 1 | 2 [20]1 [2] | 6 [22] |
| Eruptive Xanthoma | 1 | 1 [22]2 [21] | - |
| Perforating disorders | 4 | 2 [21]4 [20] | 8 [13] |
| Insulin lipodystrophy | 1 | 1 [22] | 4 [8] |
Limitation
This study was a hospital based study and was also conducted for a limited period of time with less number of patients. Therefore, we would like to look forward for larger group studies in general population to validate these findings.
Conclusion
In conclusion, we have observed that infections were the most common cutaneous manifestation, which occurred in diabetics with high HbA1C levels (>7) i.e., in uncontrolled diabetes. A good glycaemic control will definitely reduce the incidence and severity of various cutaneous manifestations of diabetes. Therefore health education regarding diabetic control can benefit the patients. Thus, role of a dermatologist is essential for early detection of potentially grave conditions and to provide prompt care for the patients thereby improving the quality of life of diabetic patients.
In study group-Total no of patients with fungal infections: 89; Total no of patients with bacterial infections: 31; N=300 represents total number of patients in study group; N=100 represents total number of patients in control group
Out of 89 patients having fungal infections 7 patients had two fungal infections. So total number of fungal infections comes to 96
When the same patient had two dermatoses under the same group, that patient had been counted for both of those dermatoses. This is the reason for the discrepancy in numbers between this table and [Table/Fig-3].
16 patients had other dermatoses like keloid (4), melasma (3), erythroderma (3), alopecia areata (3) and androgenetic alopecia (3)
When the same patient had two dermatoses under the same group, that patient had been counted for both the dermatoses
When the same patient had two dermatoses under the same group, that patient had been counted under both the dermatoses
Chi-square test was used for the comparison to calculate p-value. Level of significance was fixed as 5% (p-value <0.05)