Comparison of Filter Paper and Gelfoam as Templates for Orientation of Endoscopic Duodenal Biopsies
Priyavadhana Balasubramanian1, Bhawana Ashok Badhe2, Rajesh Nachiappa Ganesh3, Lakshmi C Panicker4, Pazhanivel Mohan5
1 Junior Resident, Department of Pathology, Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry, India.
2 Professor, Department of Pathology, Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry, India.
3 Additional Professor, Department of Pathology, Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry, India.
4 Assistant Professor, Department of Medical Gastroenterology, Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry, India.
5 Assistant Professor, Department of Medical Gastroenterology, Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Rajesh Nachiappa Ganesh, Additional Professor, Department of Pathology, Jawaharlal Institute of Post Graduate Medical Education and Research, Puducherry, India.
E-mail: drngrajesh@gmail.com
Introduction
Proper orientation of endoscopic mucosal biopsies is crucial for accurate histomorphologic assessment.
Aim
To compare the morphology on two different templates, filter paper and gelfoam for orientation of biopsies.
Materials and Methods
In this study a total of 21 duodenal biopsies were studied which were taken from the same site, fixed and oriented on wet filter paper and gelfoam in formalin, from patients who presented with malabsorptive symptoms. Histomorphological parameters studied were villous architecture, crypt architecture, intraepithelial lymphocyte (IEL) count per 100 enterocytes, villous tip IEL count per 20 enterocytes, inflammatory cells in lamina propria. Statistical analysis was done using IBM- SPSS software version 21. p-value <0.05 was considered as statistically significant.
Results
Twenty-one biopsies were taken in both the templates. Authors had 18 biopsies for analysis as three sections on gelfoam were lost during processing. All the histomorphological parameters were studied and it was similar on both the templates. The level of agreement by kappa statistics was significant with kappa value of 0.727 for villous architecture, 0.852 for lamina propria inflammatory infiltrate, 1 for IEL and eosinophils in lamina propria with p-value of <0.001.
Conclusion
Authors concluded, gelfoam or filter paper serves as a good template for proper orientation of tiny mucosal biopsies. However, further studies are needed on larger sample size to validate this finding.
Endoscopic mucosal biopsies, Gastrointestinal endoscopies, Lamina propria
Introduction
Upper gastrointestinal endoscopies are being done routinely nowadays. A non-fragmented, well-oriented biopsy specimen aids in establishing an accurate diagnosis. The optimal method of orienting the specimen is to put the base of the mucosa on filter paper and float it upside down in a bottle with formalin, allowing the villi to float freely and subsequently let the specimen with the villi to hang down in a dependent way, to minimise architectural distortion [1]. The orientation of biopsy is done by keeping the luminal side up. An obliquely cut millipore filter paper is used for multiple biopsies.
Orientation of biopsy is important as the evaluation of villous crypt architecture and the intraepithelial lymphocyte counts and distribution is critical, especially in celiac disease. Intraepithelial lymphocytes are seen interspersed between the epithelial cells. They are counted in randomly selected area where well-oriented villi are seen. So, good orientation is important in this aspect too. Some organisms colonise the enterocytes in the villous tips and hence orientation becomes important in diagnosing these conditions. Poor quality and tangential sectioning often lead to misinterpretation and misdiagnosis [2].
Artefacts which can interfere with histological interpretation are crush artefacts due to squeezing of tissues and focal haemorrhages which occur during biopsy, inadequate or improper fixation leading to poor preservation of tissue, improper processing or orientation during embedding or improper staining can result in technically poor sections, further causing difficulty in interpretation. Artefactual blunting of villi over Brunners gland and lymphoid follicles may give erroneous villous crypt ratio [3,4]. Broadening of villi occurs when the biopsy shows no muscularis mucosae [5].
Most of the artefacts can be prevented if these tiny mucosal biopsies are sent in formalin with supporting templates like filter paper, gelfoam, or cardboard. In this study, authors compared the morphology on two different templates, filter paper and gelfoam for orientation of biopsies.
Materials and Methods
In this study a total of 21 duodenal biopsies were analysed which were taken from the same site, fixed and oriented on wet filter paper and gelfoam in formalin, from patients who presented with malabsorptive symptoms. Out of 21 cases, only 18 were stained as three cases were lost during sectioning or processing. Four tiny tissue bits were taken during endoscopic biopsy, two was kept in formalin and two in gelfoam using non-toothed forceps. These tiny mucosal biopsies were fixed and oriented randomly immediately on wet filter paper of size 3 cm×2 cm (Whatman qualitative filter paper, Grade 1) and wet gelfoam of size 2 cm×2 cm (Gelfoam® absorbable gelatin sponge, USP) in penicillin bottles with formalin, using small wooden sticks with the luminal side facing upwards. Processing, paraffin embedding and section cutting were done along with gelfoam. Care was taken not to damage the sample. One section was taken for Haematoxylin and Eosin (H&E), one for special stain and two sections for IHC were taken per block.
Studied histomorphological parameters were villous architecture, crypt architecture, Intraepithelial Lymphocyte (IEL) count per 100 enterocytes (average of 300 enterocytes were taken), villous tip IEL count per 20 enterocytes counted in randomly well-oriented villi. Inflammatory cells in lamina propria: type of cells-lymphocytes, neutrophils, eosinophils, epithelioid cells and its severity was graded as ‘Mild’, ‘Moderate’ and ‘Severe’ [6]. Special stains like Giemsa, Ziehl Neelsen for acid-fast bacilli and PAS were done if indicated. Immunohistochemistry for CD 3 antibody was done for IELs.
Villous architecture was subjectively assessed and classified as normal and blunting of villi. Blunting of villi was graded as ‘Mild’, ‘Moderate’ or ‘Severe’ blunting based on subjective assessment of villous crypt architecture. Normal Villous Crypt ratio (V:C) is 3:1 to 5:1. Mild blunting was given when the ratio was 2:1, moderate blunting when the ratio was 1:1 and flattening when there were no villi seen. Also, villous atrophy was studied as absent or present and if present whether it was partial or total villous atrophy. Shorter and thicker villi are seen in areas overlying Brunners glands or lymphoid follicles so these areas were excluded. Crypt architecture was subjectively assessed as normal, hyperplastic or atrophic. Modified Marsh Oberhuber G et al., classification was used to classify Celiac disease [6].
IELs were calculated per 100 enterocytes from the average of 300 enterocytes and was graded as ‘normal’ when <25 IELS per 100 enterocytes, 25-29 as borderline increased and >29 as definitely increased [7]. IEL count was studied in both H&E stained sections and by using CD3 immunohistochemistry and was compared. IEL counts by CD3 immunohistochemistry was taken as the final but in cases where IHC for CD3 was not done IEL counts done on H&E stained section was taken as final count.
Villous tip IELs were also counted from randomly selected area showing well-oriented villi. IELs per 20 enterocytes were counted for five villi and mean counts taken in both H&E stained sections and by using CD3 immunohistochemistry. Less than five was taken as normal and more than five as definitely increased [6].
The types of cells in the lamina propria were studied and the severity was graded as mild, moderate and severely increased [6]. Eosinophils were counted per five high power field and <22 was taken as normal and >22 as increased [7].
Statistical Analysis
Statistical analysis was done using IBM-SPSS software version 21.0. The p-value <0.05 was considered as statistically significant.
Results
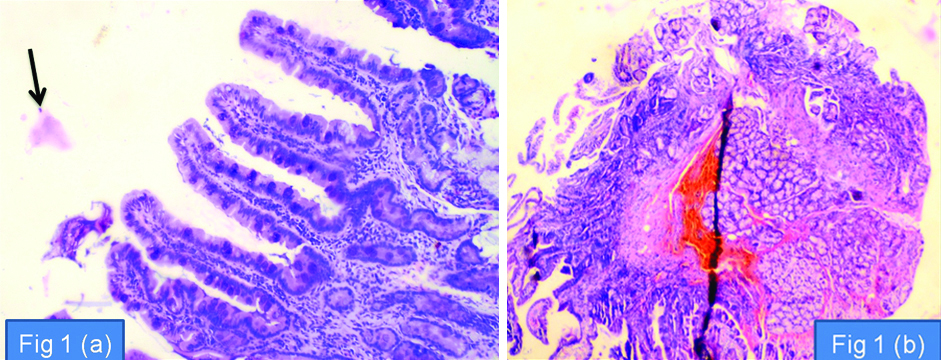
Twenty-one biopsies were taken in both the templates. Authors had only 18 biopsies for analysis as in three biopsies, sections on gelfoam were lost during processing. Authors had one case of celiac disease, one case of parasitic infestation, one case of autoimmune disease and 15 cases of non-specific duodenitis. Chattering artefacts were observed with filter paper, whereas section cutting was difficult with biopsies in gelfoam. Gelfoam remains during processing interferes with section cutting and can be seen in tissue sections and stained slides. Gelfoam led to increase in tissue loss due to repeated sectioning whereas tissue loss was insignificant when biopsies were embedded with tissue paper [Table/Fig-1a,b,2].
a) Section shows fragment of duodenal mucosa showing well-oriented villi, fixed on gelfoam template. The arrow points the gelfoam material, seen as pale pink material. (H&E 10X); b) Section shows whole view of duodenal mucosa with chattering artifact, fixed on filter paper template. (H&E 4X).
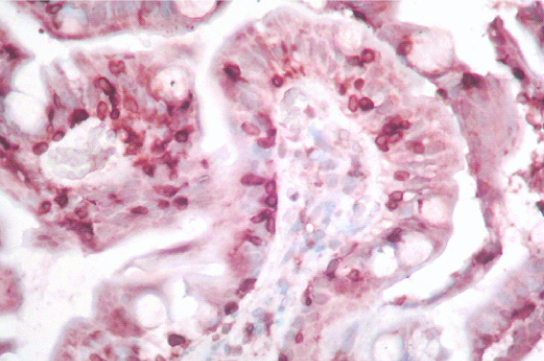
Villi showing intraepithelial lymphocytes, highlighted by CD3, IHC (40X).
The age group ranged from 19-45 years. There were seven males and 11 females. All the histomorphological parameters were studied and reported independently by three different histopathologists and it was similar on both the templates. The level of agreement by kappa statistics for villous architecture, lamina propria inflammatory infiltrate, IEL and eosinophils in lamina propria were 0.727, 0.852, 1 and 1 respectively and all were strongly significant [8]. [Table/Fig-3] shows comparison of parameters between gelfoam and filter paper.
Comparison between gelfoam and filter paper.
| Parameters | No of cases | Kappa value | p-value |
|---|
| Villous architecture |
| Normal | 14 | 0.727 | <0.001 |
| Mild blunting | 3 |
| Moderate blunting | 1 |
| Severe blunting | - |
| Villous atrophy |
| Present | 1 | 1 | <0.001 |
| Absent | 17 |
| Lamina propria inflammatory infiltrate |
| Mild | 16 | 0.852 | <0.001 |
| Moderate | 1 |
| Severe | 1 |
| Eosinophils in lamina propria |
| Increased | 1 | 1 | <0.001 |
| Normal | 17 |
| IEL count |
| Normal | 15 | 1 | <0.001 |
| Borderline increased | 2 |
| Definitely increased | 1 |
Discussion
In the present study, authors studied 18 duodenal biopsies in two different templates, filter paper and gelfoam for orientation and analysed the histomorphological parameters. Very few studies are available in literature using various templates. Auriati L et al., have used cellulose acetate millipore filters for orientation of biopsies. They studied 40 biopsies, 20 from oesophagus and 20 from gastric antrum and compared for orientation and morphology. They concluded that Millipore filters allowed better orientation of biopsy samples and also improved the diagnostic assessment [9]. However, the present authors studied duodenal biopsies and found similar findings on two different templates.
Ruiz GC et al., studied biopsy specimens taken from the stomach and duodenum from dogs and cats using three different templates, mounted on cucumber slice, moisturised synthetic foam sponge and floating free in formalin and they concluded that the use of mounted gastrointestinal biopsy specimens was superior to that of specimens floating free in formalin. This further, improved the quality of the specimens and better histomorphologic interpretation [10].
Veitch AM and Fairclough PD, from Homerton and SL Bartholomews Hospital described a simple, cheap and reliable method of presenting multiple biopsies to facilitate easy handling of the specimens. They used cellulose acetate electrophoresis paper to mount endoscopic specimens. They also found that there was good initial adhesion of specimens to the cellulose acetate paper [11].
Limitation
The study was done on small number of samples. Further studies are needed on larger sample size to validate this finding. Filter paper was a cost effective alternative for gelfoam. However, there were chattering artefacts with tissue paper while tissue loss was greater in gelfoam. Section cutting was also difficult with biopsies in gelfoam.
Conclusion
Authors compared gelfoam and filter paper for orientation of endoscopic duodenal biopsies and conclude that both gelfoam and filter paper serves as a good template for proper orientation of tiny mucosal biopsies.
[1]. Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Lantz PE, Isaacson PG, The Non Neoplastic Small Intestine. In: Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Lantz PE, Isaacson PG. (eds.)Gastrointestinal Pathology: An Atlas and Text 2008 3 EdPhiladelphia, PennsylvaniaWalterKluvers, Lippincott, Williams & Wilkins publishing:409 [Google Scholar]
[2]. Shidrawi RG, Przemioslo R, Davies DR, Tighe MR, Ciclitira PJ, Pitfalls in diagnosing coeliac diseaseJ Clin Pathol 1994 47(8):693-94.10.1136/jcp.47.8.6937962617 [Google Scholar] [CrossRef] [PubMed]
[3]. Perera DR, Weinstein WM, Rubin CE, Symposium on pathology of the gastrointestinal tract-Part II. Small intestinal biopsyHum Pathol 1975 6:157-217.10.1016/S0046-8177(75)80176-6 [Google Scholar] [CrossRef]
[4]. Petras RE, Nonneoplastic Intestinal Diseases. In Mills SE, Carter D, Greenson JK, Obernman HA, Reuter V, Stoler MH. (eds.)Sternberg’s Diagnostic Surgical Pathology 2004 4 edPhiladelphiaLippincott Williams & Wilkins:1475-1542. [Google Scholar]
[5]. Whitehead R, Mucosal biopsy of the gastrointestinal tract. In: Bennington JL. (ed.)Major Problems in Pathology 1985 Vol 33EdPhiladelphiaWB Saunders [Google Scholar]
[6]. Oberhuber G, Granditsch G, Vogelsang H, The histopathology of coeliac disease: time for a standardized report scheme forpathologistsEur J Gastroenterol Hepatol 1999 11:1185-94.10.1097/00042737-199910000-0001910524652 [Google Scholar] [CrossRef] [PubMed]
[7]. Walker MM, Salehian SS, Murray CE, Rajendran A, Hoare JM, Negus R, Implications of eosinophilia in the normal duodenal biopsy-an association with allergy and functional dyspepsiaAliment Pharmacol Ther 2010 31(11):1229-36.10.1111/j.1365-2036.2010.04282.x20222916 [Google Scholar] [CrossRef] [PubMed]
[8]. McHugh ML, Interrater reliability: the kappa statisticBiochem Med (Zagreb) 2012 22(3):276-82.10.11613/BM.2012.03123092060 [Google Scholar] [CrossRef] [PubMed]
[9]. Auriati L, Truini M, Sebastiani P, Bruzzone G, Fiocca R, Use of cellulose acetate millipore filters for the correct orientation of endoscopic biopsies in digestive diseasesPathologica 2003 95:146-51. [Google Scholar]
[10]. Ruiz GC, Reyes-Gomez E, Hall EJ, Freiche V, Comparison of 3 Handling Techniques for Endoscopically Obtained Gastric and Duodenal Biopsy Specimens: A Prospective Study in Dogs and CatsJ Vet Intern Med 2016 30:1014-21.10.1111/jvim.1440327396683 [Google Scholar] [CrossRef] [PubMed]
[11]. Veitch AM, Fairclough PD, An improved method of handling endoscopic biopsy specimensGastrointestinal Endoscopy 1995 41:18310.1016/S0016-5107(05)80621-2 [Google Scholar] [CrossRef]