Case Report 1
A 55-year-old female patient who was admitted in respiratory medicine unit of Chhatrapati Shivaji Subharti Hospital, Meerut, Uttar Pradesh, India, with complaints of severe breathlessness, high grade fever since a week and productive cough. She was apparently well five years back when she developed productive cough and dyspnoea with seasonal variation. She gives history of fever with chills and rigor off and on since last eight months and the patient related a history of weight loss (19 kg in these 5 years), productive cough and swelling in lower limb since one month.
On clinical examination, the patient was thin built and emaciated and her present weight was 30 kg. Her pulse rate was 110/min, blood pressure was 110/70 mm of Hg and the body temperature was 100°F. There was mild pallor and pedal oedema but no icterus, cyanosis and clubbing. Examination of the respiratory system revealed a respiratory rate of 25/min, with oxygen saturation 85% and there were bilateral infra-clavicular and inframammary crepitations. Her haemoglobin was 9.5 g%, total leucocyte count was 25,500/cu mm with differential leucocyte counts showing 90% of neutrophils and 10% of lymphocytes and erythrocyte sedimentation rate of 84 mm/h. The liver function test was within normal range and in kidney function tests blood urea was 67 mg/dL and creatinine was 1.2 mg/dL. Arterial blood gas analysis was suggestive of respiratory alkalosis. Her fasting blood sugar was 257 mg/dL suggesting uncontrolled diabetes. Antibodies to HIV-1 and 2 were non-reactive.
The chest radiograph postero-anterior view revealed bilateral heterogenous opacities with bilateral hila pulled upwards, pleural thickening and pleural effusion, suggestive of pulmonary tuberculosis. To confirm Zeihl Neelson stained sputum smear was sent to DOTS section for microscopy which revealed acid fast bacilli i.e., pink coloured, slender and slightly curved bacilli (grade:1+) against blue background, suggestive of Mycobacterium tuberculosis, following which ATT was started. Ultrasonographic colour doppler revealed Deep Vein Thrombosis (DVT) in lower limb.
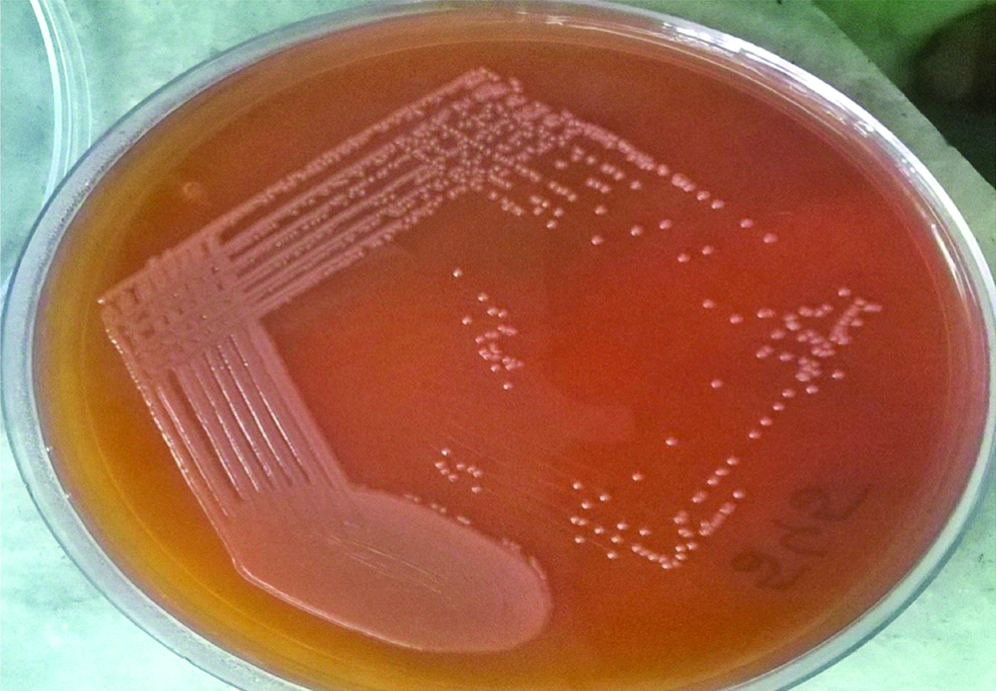
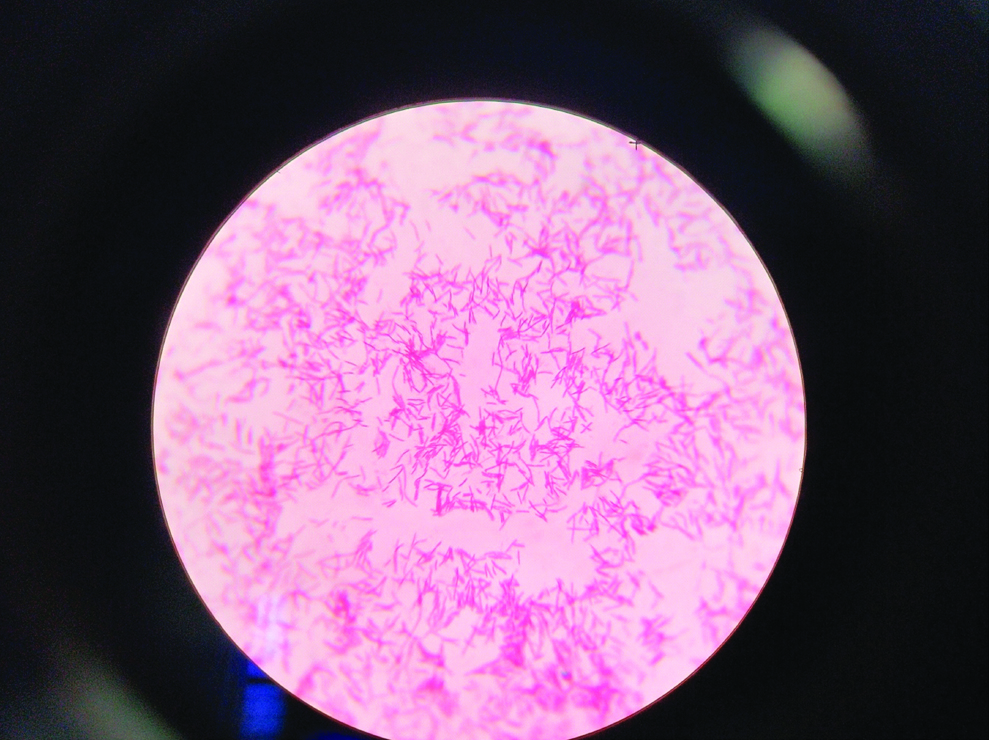
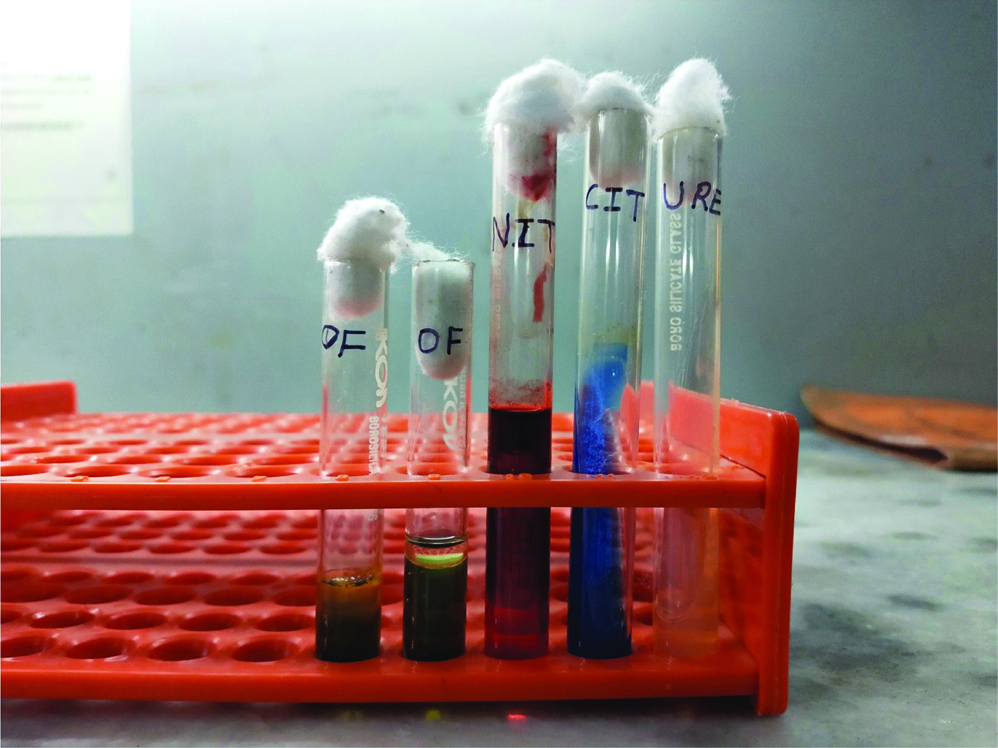
Simultaneously, on the day of admission her sputum sample was received in clinical microbiology laboratory. Gram stain of sputum revealed plenty of polymorphonuclear leukocytes and mixed flora. The sample was cultured on blood agar, chocolate agar and Mac Conkey agar plates and incubated at 37°C overnight. Smooth grayish white colonies were observed on blood agar and chocolate agar plates. Mac Conkey agar plate showed non-lactose fermenting colonies [Table/Fig-1]. The isolate was motile, catalase and oxidase positive. Culture smear revealed Gram negative bacilli which were predominantly straight while few were curved and club shaped [Table/Fig-2]. On biochemical analysis the isolate utilised citrate, reduced nitrates to nitrites, showed oxidative reaction in Hugh Leifson’s Oxidation/Fermentation media for glucose and xylose along with decarboxylation of lysine and ornithine [Table/Fig-3]. However, urea was not hydrolysed, indole was not produced and arginine was not hydrolysed. The above finding alerted us of growth of some unusual pathogen which required further confirmation, by VITEK® 2 system (biomerieux, France) using GN REF 21341 card which identified the isolate as A. xylosoxidans. The drug susceptibility of the isolate was also performed using VITEK® 2 AST-N281 REF 414532 card. Both the sputum samples received on two consecutive days grew A. xylosoxidans with similar sensitivity pattern. The isolates were sensitive to piperacillin, piperacillin-tazobactum, ciprofloxacin, aminoglycosides, carbapenems and colistin but resistant to ceftazidime, cefepime and aztreonam. However, we could not isolate A. xylosoxidans from blood culture which we received the next day. Following this, the patient was started on intravenous piperacillin-tazobactum for two weeks. Follow-up done after completion of two weeks therapy showed significant improvement in patient’s condition and repeat sputum culture done was negative for growth of A. xylosoxidans.
Mac Conkey agar showing non-lactose fermenting colonies.
Gram stain of culture smear showing Gram negative bacilli predominantly straight, few curved and club shaped.
Results of the biochemical tests.
Case Report 2
A 67-year-old male patient who was admitted in the emergency unit of our hospital with complaints of severe breathlessness, high grade fever and productive cough since 15 days. He was apparently well two months back when he developed productive cough and dyspnea. He gave history of on and off fever with chills and rigor since last two months.
On clinical examination, the patient was thin built. His pulse rate was 106/min, blood pressure was 102/68 mm of Hg and the body temperature was 101°F. There was mild pallor but no icterus, cyanosis, clubbing. Examination of the respiratory system revealed, respiratory rate was 28/min, with oxygen saturation 82%. His haemoglobin was 10.2 g%; total leucocyte count was 22,300/cu mm with differential leucocyte counts showing 88% of neutrophils and 12% of lymphocytes and erythrocyte sedimentation rate of 78 mm/h. The liver function tests were within normal range and in kidney function tests blood urea was 71 mg/dL and creatinine was 1.2 mg/dL. Arterial blood gas analysis was suggestive of respiratory alkalosis. Her fasting blood sugar was 87 mg/dL. Antibodies to HIV-1 and 2 were non-reactive.
The chest radiograph postero-anterior view revealed increased vascular markings and cardiomegaly. On the lateral radiograph, a “barrel chest” with widened anterior-posterior diameter was seen. CT of chest revealed bronchovascular irregularity and fibrosis and diagnosed as a case of COPD.
On the day of admission, sputum for culture and blood culture were received in clinical microbiology laboratory. Gram stain of sputum revealed plenty of polymorphonuclear leukocytes with plenty of gram negative bacilli. The sample was cultured on blood agar, chocolate agar and Mac Conkey agar plates and incubated at 37°C overnight. Blood culture bottle was incubated in automated BacT/ALERT 3D.
After overnight incubation, smooth grayish white colonies were observed on blood agar and chocolate agar plates in sputum and Macconkey agar plate showed non-lactose fermenting colonies. The isolate was motile, catalase and oxidase positive. Culture smear revealed Gram negative bacilli which were predominantly straight while few were curved and club shaped.
BacT/ALERT 3D showed positive signal for bacterial growth within 24 hours of incubation which showed Gram negative bacilli and the clinician was alerted immediately. The blood culture grew similar type of colonies to that of sputum and the isolate was motile, catalase and oxidase positive.
On biochemical analysis both the isolates utilised citrate, reduced nitrates to nitrites, showed oxidative reaction in Hugh Leifson’s Oxidation/Fermentation media for glucose and xylose along with decarboxylation of lysine and ornithine. However, urea was not hydrolysed, indole was not produced and arginine was not dihydrolysed. The above findings alerted us of growth of some unusual pathogen thus for further confirmation, we tested by VITEK® 2 system (biomerieux, France) using GN REF 21341 card which identified the isolates as A. xylosoxidans. The drug susceptibility of the isolates was also performed using VITEK® 2 system by AST-N281 REF 414532 card. Both the samples (sputum and blood) grew A. xylosoxidans with similar sensitivity pattern. The isolates were sensitive to piperacillin, piperacillin-tazobactum, ciprofloxacin, aminoglycosides, carbapenems and colistin but resistant to ceftazidime, cefepime and aztreonam. Following this, the patient was started on intravenous piperacillin-tazobactum for 21 days. Follow-up done after completion of 21 days therapy showed drastic improvement in patient’s condition. Repeat sputum and blood culture done after 21 days were negative for growth of A. xylosoxidans.
Discussion
A. xylosoxidans is a saprophyte found in soil, intravenous fluids, water in wells and humidifiers. It is recently emerging as an opportunistic pathogen [1,2]. A. xylosoxidans causes infections in both immunocompetent and immunocompromised hosts, which include bacteraemia, pneumonia, meningitis, abscesses, urinary tract infections, osteomyelitis, endocarditis and peritonitis [2].
It is an aerobic, non-fermenting, motile, oxidase positive Gram-negative bacilli [3]. It was also called Alcaligenes xylosoxidans [4]. Yabuuchi E et al., first identified and described it in 1971, from seven cases of chronic otitis media, belongs to family Alcaligenaceae and genus Achromobacter [5]. The most clinically significant subspecies are Achromobacter xylosoxidans and denitrificans [6].
Immunocompromised status and/or prior structural damage play a role in the pathogenicity of A.xylosoxidans. In the present cases, the source of acquisition of pulmonary infection could be environmental, as these patients visited local practitioner in rural area and was put on oxygen inhalation, nebulization and intravenous fluids off and on. Thus, they might have acquired the pathogen through humidifier during oxygenation (nebulization).
It has been observed that immunosuppressed population is at higher risk of infection due to Achromobacter species [1]. Similar finding was observed in our cases as our first patient was suffering from pulmonary tuberculosis with diabetes mellitus and second patient had COPD, emphasising that the damaged lung tissue was the predisposing factor for infection with this organism. Virulence factors of Achromobacter species are biofilm formation and motility (via pili and flagella) [1].
Many cases of A. xylosoxidans have being isolated from various clinical samples and have been reported both from India and abroad [1,7]. Cases of pneumonia, a case of septic arthritis by Suryavanshi KT et al., [3]. Similarly first pancreatic pseudocyst and local wound infection of metastatic ductal carcinoma was reported by Eshwara VK et al., [4] and perinephric abscess case reported by Vinod V et al., from India [8]. Other reported infections in adults include meningitis [9], endocarditis [10], peritoneal dialysis and catheter related peritonitis [11].
A. xylosoxidans can be confused with other non-fermentative, oxidase positive, motile gram-negative rods, especially Pseudomonas species, in clinical specimens and hence identification of this emerging pathogen can be missed which can affect the clinical management of patient substantially [12]. Automation played an important role in rapid and correct identification of such pathogens and hence timely initiation of appropriate antibiotic therapy in our cases led to a favourable outcome.
Both the patients showed similar sensitivity pattern and were treated with intravenous piperacillin-tazobactum for duration of 2-3 weeks. Rapid diagnosis and prompt treatment saved the lives of both the patients.
Conclusion
The cases highlight that, A. xylosoxidans can cause respiratory infections, especially among immunocompromised patients with underlying lung disease. The organism occurs as a saprophyte in soil and water environment. It is an oxidase positive, motile, gram negative non fermenter which can be confused with Pseudomonas species. Automation helps in correct and rapid identification of such rare pathogens. Rare causes of pulmonary infections should be investigated carefully since appropriate detection can facilitate accurate antibacterial management.
[1]. Awadh H, Mansour M, Aqtash O, Shweihat Y, Pneumonia due to a rare pathogen: Achromobacter xylosoxidans, subspecies denitrificansCase Reports in Infectious Diseases 2017 2017:396968210.1155/2017/396968228894613 [Google Scholar] [CrossRef] [PubMed]
[2]. Duggan JM, Goldstein SJ, Chenoweth CE, Kauffman CA, Bradley SF, Achromobacter xylosoxidans bacteraemia: Report of four cases and review of the literatureClinical Infectious Diseases 1996 23:569-76.10.1093/clinids/23.3.5698879782 [Google Scholar] [CrossRef] [PubMed]
[3]. Suryavanshi KT, Lalwani SK, Uncommon pathogen: Serious manifestation: A rare case of Achromobacter xylosoxidans septic arthritis in immunocompetent patientIndian J Pathol Microbiol 2015 58:395-97.10.4103/0377-4929.16292926275277 [Google Scholar] [CrossRef] [PubMed]
[4]. Eshwara VK, Mukhopadhyay C, Mohan S, Prakash R, Pai G, Two unique presentations of Achromobacter xylosoxidans infections in clinical settingsJ Infect Dev Ctries 2011 5:138-41.10.3855/jidc.125821389595 [Google Scholar] [CrossRef] [PubMed]
[5]. Yabuuchi E, Yano I, Goto S, Tanimura E, Shiito T, Ohyama A, Description of Achromobacter xylosoxidansInt J Syst Evol Microbiol 1974 24:470-77.10.1099/00207713-24-4-470 [Google Scholar] [CrossRef]
[6]. Coenye T, Vancanneyt M, Falsen E, Swings J, Vandamme P, Achromobacter insolitus sp. nov. and Achromobacter spanius sp. nov., from human clinical samplesInternational Journal of Systematic and Evolutionary Microbiology 2003 53:1819-24.10.1099/ijs.0.02698-014657110 [Google Scholar] [CrossRef] [PubMed]
[7]. Bharadiya A, Mane M, Pawar S, Aundhakar S, “Watch Out! pneumonia secondary to Achromobacter denitrificans”Annals of Medical and Health Sciences Research 2014 4:2210.4103/2141-9248.13170025031900 [Google Scholar] [CrossRef] [PubMed]
[8]. Vinod V, Kumar A, Sanjeevan KV, Dinesh KR, Karim S, Perinephric abscess due to Achromobacter xylosoxidans following de-roofing of renal cystSurg Infect (Larchmt) 2013 14:422-23.10.1089/sur.2012.14223859686 [Google Scholar] [CrossRef] [PubMed]
[9]. Manckoundia P, Mazen E, Coste AS, A case of meningitis due to Achromobacter xylosoxidans denitrificans 60 years after a cranial traumaMedical Science Monitor 2011 17:CS63-CS65.10.12659/MSM.88179621629191 [Google Scholar] [CrossRef] [PubMed]
[10]. Derber C, Elam K, Forbes BA, Bearman G, Achromobacter species endocarditis: A case report and literature reviewCanadian Journal of Infectious Diseases and Medical Microbiology 2011 22:e17-e20.10.1155/2011/52741222942890 [Google Scholar] [CrossRef] [PubMed]
[11]. Cankaya E, Keles M, Gulcan E, Uyanik A, Uyanik H, A rare cause of peritoneal dialysis-related peritonitis: Achromobacter denitrificansPeritoneal Dialysis International 2014 34:135-37.10.3747/pdi.2013.0006324525606 [Google Scholar] [CrossRef] [PubMed]
[12]. Siegman VI, Chmel H, Cobbs C, Clinical and laboratory characteristics of Achromobacter xylosoxidans infectionJournal of Clinical Microbiology 1980 11:141-45. [Google Scholar]