Hypertensive disorder of pregnancy is a leading cause of maternal and perinatal mortality and morbidity worldwide with an incidence rate of 5-10% of all pregnancies [1]. Preeclampsia is a severe form which has multisystem involvement and fatal complication [1,2]. The main management of preeclampsia with severe features are hospitalisation, seizure prevention, control blood pressure and termination of pregnancy. Magnesium Sulphate (MgSO4) is the most effective drug used for seizure prevention [3,4], as this drug can reduce the risk of eclampsia by more than 50% [5,6]. The dosage of MgSO4 is still in diverse however, two commonly used MgSO4 infusions protocols are intravenous (Zuspan regimen) [7] and intramuscular route (Pritchard regimen) [8]. For the intravenous regimen, the recommended dose is 4-6 g intravenous loading dose, followed by the maintenance dose of 1 to 3 g/hr until 24 hours after delivery [7]. For the intramuscular regimen, the dosage is 4 g intravenous with 10 g intramuscular loading dose, then 5 g intramuscular every four hours [8]. The therapeutic serum level of MgSO4 for convulsion prevention is not clearly established, however the recommended serum level, based on a retrospective study, is 4.8 to 8.4 mg/dL which has been used by many centres for safe monitoring of this drug [1,9].
Materials and Methods
This study was a randomised controlled trial which was conducted from January 2018 to September 2018 at Udonthani Hospital, Thailand. The study was conducted after the approval by the Udonthani Research Ethics Committee (number 1/2561). Pregnant women with preeclampsia with severe features who met the inclusion criteria were counselled and invited to participate in this study. An informed consent was obtained after explanation of the study’s method and side effects on the participants. The parent or legal guardian provided their written informed consent for all patients under the age of 18.
The inclusion criteria were pregnant preeclamptic women with severe features who were admitted with gestational age ≥24 weeks. The preeclampsia was diagnosed according to American College of Obstetrics and Gynaecology 2013 guideline. Criteria includes systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg with proteinuria >300 mg in 24 hours or urine protein-creatinine ratio >0.3 or proteinuria ≥1+ by urine protein dipstick. Severe features were defined, as the patients who had one of the following; systolic blood pressure ≥160 mmHg or diastolic blood pressure ≥110 mmHg, serum creatinine >1.1 mg/dL or ≥2 time of baseline, transaminase levels ≥2 time of upper normal limit, platelets <100,000/μL, pulmonary oedema, cerebral symptoms such as persistent headache, visual disturbances and right upper abdominal pain [15].
The exclusion criteria were participants, who had serum creatinine >1.3, hypersensitivity to MgSO4, myocardial damage, diabetic coma, heart block and myasthenia gravis. All participants were randomly allocated into two groups using block randomisation by computer generated random number which were sealed in opaque envelops. Study and control drugs were prepared by ward nurses according to the study protocol. All patients received 4 g intravenous loading dose, and then the study group received a maintenance dose of MgSO4 by weight-adjusted protocol according to our previous report [14]. The weight-adjusted protocol was 1.2 g/hr for maternal bodyweight <60 kg, 1.3 g/hr for maternal bodyweight 60-79.9 kg, 1.4 g/hr for maternal bodyweight 80-99.9 kg and 1.5 g/hr for maternal bodyweight ≥100 kg. The control group received 2 g/hr maintenance dose [1]. The serum Mg level was monitored at two hours after loading dose and then every four hours and dosage of MgSO4 was adjusted until the therapeutic level of serum Mg was reached and then continued to 24 hours after delivery.
The blood samples were kept in a collection tube and sent to the laboratory. The serum Mg level was measured by the Arsenazo method and the creatinine level was measured by the enzymatic method [16,17]. The creatinine result was then used to calculate the Glomerular Filtration Rate (GFR) using the CKD-EPI GFR calculator program. Both tests were done by an ARCHITECT machine modelC1600 (ABBOTT). All participants were monitored for clinical magnesium toxicity such as loss of patellar reflex, oliguria, apnea and drowsiness. A 10% calcium gluconate was made available in all cases.
The characteristics of patients in both groups were recorded such as age, gravida, parity, body weight and height. The body mass index and mean arterial pressure in both groups were calculated and then compared between groups. Maternal outcomes such as mortality, eclampsia, intracranial haemorrhage, Disseminated Intravascular Coagulopathy (DIC), cardiomyopathy, Intensive Care Unit (ICU) admission, postpartum haemorrhage were recorded. Neonatal outcomes such as preterm birth, intrauterine growth retardation, low birth weight, abruption placenta, still birth, death foetus in utero and Neonatal Intensive Care Unit (NICU) admission were recorded. The serum Mg levels at two and four hours were compared between the two groups. The primary outcome measurement was the proportion of participants who achieved therapeutic level at four hours after the loading dose, and secondary outcomes were the comparison of maternal and neonatal complications between the two protocols.
Statistical Analysis
The sample size was calculated using the formula for randomised controlled trial for binary data [18]. The proportion of successful outcome from the pilot study data in the study group was 0.20, in the control group was 0.45. A α was 0.1 and the power was 80%. The calculated sample size was 43 participants in each group. A total number of 86 participants, with 43 per group, was used. The participants’ characteristics are presented in number, percentage, range or mean±standard deviation. The groups were compared using an unpaired Student’s t-test for continuous variables. Chi-square and Fisher’s-exact tests were used for categorical variables. The mean difference and relative risk with a 95% CI was calculated for the magnitude of effect. Statistical analysis was performed using Stata version13. A p-value <0.05 was considered statistically significant.
Results
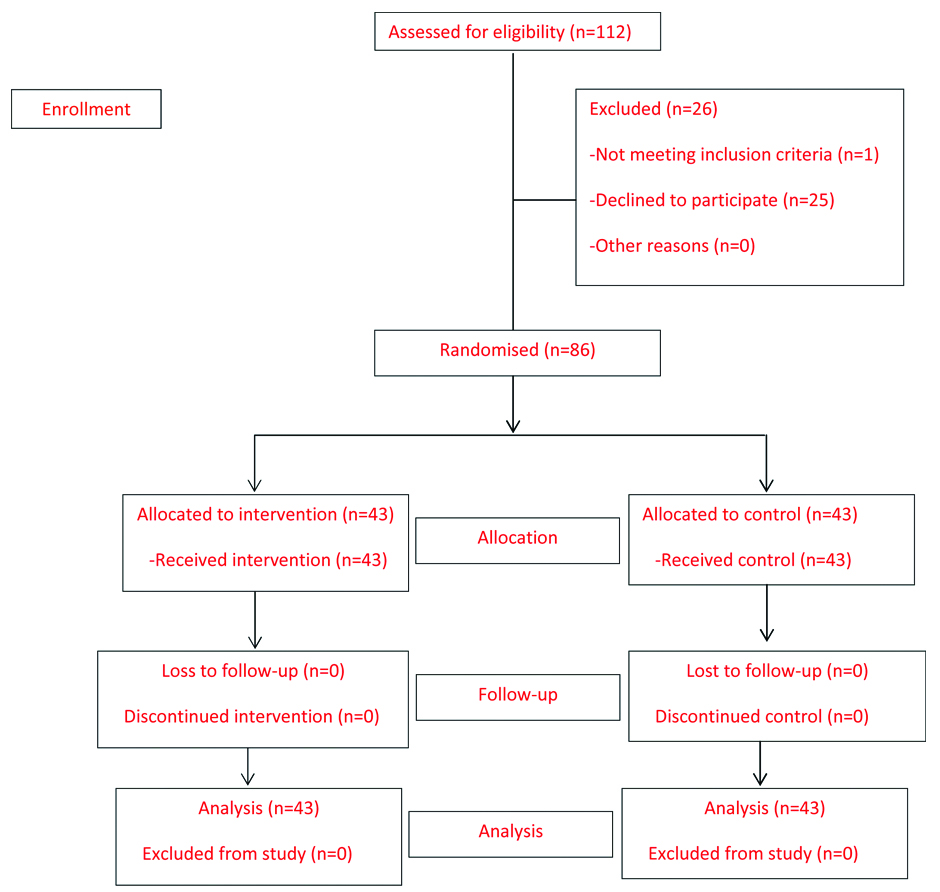
A total of 86 participants were included in this study with 43 in each group. All continued until the study completed and were included in the data analysis [Table/Fig-1]. The participants’ characteristics in both groups are shown in [Table/Fig-2]. The mean maternal bodyweight was 76.5 kg (range 50.3-126 kg). Both groups were comparable in terms of age, gestational age, body mass index, mean arterial pressure, glomerular filtration rate, serum creatinine, serum transaminase, platelet count, maternal medical disease and maternal complications such as pulmonary oedema, cerebral or visual symptoms except gravida and parity.
Comparison of epidemiological characteristics, data was presented in term of mean±standard deviation unless specified otherwise.
| Characteristics | Weight-adjusted Group (n=43) | 2 grams/hour Group (n=43) | p-value |
|---|
| Age (year), range | 29.2±7.3 (16-44) | 27.8±7.3 (15-42) | 0.41 |
| Primigravida, n (%) | 13 (30.2%) | 22 (51.2%) | <0.05*(a) |
| Nullipara, n (%) | 18 (41.9%) | 29 (67.4%) | 0.02*(a) |
| Gestational age (weeks), range | 36.4±3.2 (27-41) | 36.1±3.5 (26-41) | 0.75 |
| BMI (kg/m2) | 31.9±5.5 | 30.2±5.8 | 0.18 |
| <18.5, n (%) | 0 | 0 | 0.32(b) |
| 18.5 to <25, n (%) | 3 (7.0%) | 9 (20.9%) | |
| 25 to <30, n (%) | 15 (34.9%) | 13 (30.2%) | |
| 30 to <40, n (%) | 23 (53.5%) | 19 (44.2%) | |
| ≥40, n (%) | 2 (4.7%) | 2 (4.7%) | |
| MAP (mmHg) | 128.5±9.18 | 127.9±10.6 | 0.79 |
| GFR (mL/min) | 174.0±55.6 | 173.1±50.7 | 0.94 |
| Serum creatinine >1.1 mg/dL | 1 (2.3%) | 0 | 1.00(a) |
| Platelets < 100,000/μL | 2 (4.7%) | 2 (4.7%) | 1.00(a) |
| Transaminase levels ≥2 time of upper normal limit | 4 (9.3%) | 2 (4.7%) | 0.68(a) |
| Pulmonary oedema | 1 (2.3%) | 1 (2.3%) | 1.00(a) |
| Cerebral or visual symptoms | 10 (23.3%) | 7 (16.3%) | 0.42(a) |
| Medical disease | | | |
| Chronic hypertension | 7 (16.3%) | 5 (11.6%) | 0.53(a) |
| Gestational DM | 6 (14.0%) | 6 (14.0%) | 1.00(a) |
| Autoimmune disease | 0 | 1 (2.3%) | 1.00(a) |
p-value was calculated by Student t-test, Pearson chi-square (a) or Fisher-exact test (b);
*statistical significance (p-value<0.05); MAP: Mean arterial pressure; GFR: Glomerular filtration rate; DM: Diabetes mellitus
The primary outcome was a comparison of the effectiveness of achieving the therapeutic level between both groups. At two hours after loading dose, the mean serum Mg was not significantly different between both groups. The proportion of participants who achieved therapeutic level was 10.8% in the study group and 28.2% in the control group.
At four hours after the loading dose, the mean serum Mg was higher in the control group (mean difference 0.63, 95% CI 0.27 to 0.98, p-value<0.01). The proportion of patients who achieved therapeutic level was 11.6% in the study group and 41.9% in the control group (RR 0.28, 95% CI 0.11 to 0.68, p-value <0.01). Supra-therapeutic level was not detected in either group [Table/Fig-3].
Comparison of mean serum Mg and percentage of achieved therapeutic level in weight-adjusted and 2 grams/hour group.
| Outcome | Weight-adjusted n (%) | 2 grams/hour n (%) | RR or difference | 95% CI | p-value |
|---|
| Two hours after loading | | | | | |
| Mean serum Mg (g/dL) | 3.97±0.61 | 4.20±0.88 | 0.23 | -0.12 to 0.57 | 0.20 |
| Therapeutic level | 4 (10.8%) | 11 (28.2%) | 0.38 | 0.13 to 1.10 | 0.07 |
| Subtherapeutic level | 33 (89.2%) | 28 (71.8%) | 1.24 | 0.99 to 1.56 | 0.06 |
| Supra-therapeutic level | 0 | 0 | NA | NA | NA |
| Four hours after loading | | | | | |
| Mean serum Mg (g/dL) | 4.00±0.68 | 4.63±0.95 | 0.63 | 0.27 to 0.98 | <0.01* |
| Therapeutic level | 5 (11.6%) | 18 (41.9%) | 0.28 | 0.11 to 0.68 | <0.01* |
| Subtherapeutic level | 38 (88.4%) | 25 (58.2%) | 1.52 | 1.15 to 2.00 | <0.01* |
| Supra-therapeutic level | 0 | 0 | NA | NA | NA |
p-value was calculated by linear or logistic regression analysis; *statistical significance (p-value<0.05); There was some missing data at 2 hours after loading
According to maternal bodyweight, 11 patients were in <60 kg group, 42 patients were in 60-79.9 kg group, 27 patients were in 80-99.9 kg group and 6 patients were in ≥100 kg group. The proportions of subtherapeutic level of MgSO4 at two and four hours were higher in the weight-adjusted regimen especially in the maternal weight <80 kg. In the maternal bodyweight between 60-79.9 kg at four hours, the relative risk was 2.16, 95% CI 1.23 to 3.79, p-value <0.01. The high maternal weight (≥80 kg), in both regimens had very high proportion of subtherapeutic level of MgSO4 with 92% in 80-99.9 kg and 100% in ≥100 kg [Table/Fig-4].
Comparison of percentage of subtherapeutic level in weight-adjusted and 2 grams/hour group in different weight groups.
| Outcome | Weight-adjusted, n (%) | 2 grams/hour, n (%) | RR | 95% CI | p-value |
|---|
| BW<60 kg |
| Subtherapeutic level at two hours | 2 (50.0%) | 3 (42.86%) | 1.17 | 0.32 to 4.28 | 0.82 |
| Subtherapeutic level at four hours | 3 (75.0%) | 2 (28.57%) | 2.63 | 0.72 to 9.64 | 0.15 |
| BW 60-79.9 kg |
| Subtherapeutic level at two hours | 18 (81.82%) | 13 (65.00%) | 1.26 | 0.86 to 1.84 | 0.23 |
| Subtherapeutic level at four hours | 19 (86.36%) | 9 (42.11%) | 2.16 | 1.23 to 3.79 | <0.01* |
| BW 80-99.9 kg |
| Subtherapeutic level at two hours | 10 (71.43%) | 9 (69.23%) | 1.03 | 0.63 to 1.69 | 0.90 |
| Subtherapeutic level at four hours | 13 (92.86%) | 12 (92.31%) | 1.01 | 0.81 to 1.25 | 0.96 |
| BW≥100 kg |
| Subtherapeutic level at two hours | 3 (100%) | 3 (100%) | NA | NA | NA |
| Subtherapeutic level at four hours | 3 (100%) | 3 (100%) | NA | NA | NA |
BW: Body weight; p-value was calculated by logistic regression analysis; *statistical significance (p-value<0.05)
The pregnancy outcome include maternal complications, route of delivery, neonatal complications shown in [Table/Fig-5]. No Mg toxicity or eclampsia was detected during study period. Maternal complications, route of delivery and neonatal complications were not significantly different between both groups.
| Variables | Weight-adjusted | 2 grams/hour | p-value |
|---|
| Magnesium toxicity | | | |
| Loss of patellar reflex | 0 | 0 | NA |
| Respiratory paralysis | 0 | 0 | NA |
| Cardiac arrest | 0 | 0 | NA |
| Maternal complication | | | |
| Convulsion | 0 | 0 | NA |
| ICU admission | 2 (4.7%) | 2 (4.7%) | 1.00 |
| Route of delivery | | | |
| Normal labour | 10 (23.3%) | 17 (39.5%) | 0.12 |
| Caesarean section | 31 (72.1%) | 26 (60.5%) | |
| Vacuum extraction | 2 (4.7%) | 0 | |
| Neonatal complication | | | |
| NICU admission | 4 (9.52%) | 5 (12.2%) | 0.70 |
| Perinatal death | 0 | 0 | NA |
ICU: Intensive Care Unit; NICU: Neonatal Intensive Care Unit; p-value was calculated by Pearson chi-square or Fisher-exact test; statistical significance (p-value<0.05)
Discussion
Magnesium sulfate is proven to be the first line drug to prevent seizure in preeclamptic women. However, the proper dosage of this drug has not been clearly established. Many centres use the Zuspan regimen by MgSO4 4-6 g intravenous loading dose, followed by an infusion of the maintenance dose of 1-2 g/hr. The maintenance dose was adjusted until the therapeutic level was reached then continued until 24 hours after delivery [7]. The weight-adjusted maintenance dose in this study was derived from a retrospective analysis of optimum maintenance dose which achieved therapeutic level of serum Mg in preeclamptic women [14].
The comparison of both protocols in this study showed no eclampsia in either protocol. Also the maternal bodyweight had a significant effect on the serum Mg level. Many studies reported a high proportion of subtherapeutic level of MgSO4 in 4 g loading and 1 g/hr maintenance [12,13,19]. This study also shows with either a 2 g/hr or a weight-adjusted maintenance dose there still had a high proportion of subtherapeutic level of MgSO4, especially in those with high maternal bodyweight. The proportion of those who achieved therapeutic level at four hour in the weight-adjusted group in this study was lower than the previous studies which used the 1 g/hr regimen [10,20]. This might be due to the proportion of high maternal bodyweight participants in this study, most of these patients had their BMI ≥30.
The proportions of participants in the study group who achieved therapeutic level in high maternal weight subgroups were 0-7.2%. This is compatible with the previous study in which high body mass index subgroup (≥30 kg/m2) achieved therapeutic level in 7.4% [20]. The result of the 2 g/hr protocol from this study also had only 40.5% of participants who achieved therapeutic level. This is lower than a previous study that reported 80% of the participants who achieved therapeutic level, supra-therapeutic level was reported in 13% in the previous study but none were found in this study [10]. Other MgSO4 clinical toxicities such as loss of patella reflex or respiratory depression were also not detected in this study.
This study is first to report the weight-adjusted protocol of MgSO4 by prospective randomised controlled trial. The block randomisation with opaque envelope made for a good representation of the population.
Limitation
There are some limitations to this study. The first is the sample size was calculated from the proportion of participants who achieved a serum therapeutic level of MgSO4, not the incidence of eclampsia. So the effectiveness of MgSO4 in eclampsia prevention needs a larger sample size to be conclusive. Second, the sample size in some maternal bodyweight groups, such as <60kg, was small, a larger sample size is needed for the specific recommended dose for lower weight patients with preeclampsia. Moreover, the loading dose in this study was 4 g, the effect of higher loading dose to the serum Mg still needs further evaluation.
The study implies that 2 g/hr regimen for maintenance dose is better than a lower dose regimen, however further researches study, for weight-adjusted dose in high maternal weight preeclamptic patients, are needed.
Conclusion
Maternal bodyweight is important for MgSO4 dosage adjustment. When the maternal bodyweight was <80 kg, a 2 g/hr maintenance dose was more effective than a lower dose. However, when the maternal bodyweight was ≥80 kg, a higher dose than 2 g/hr was required for achieving therapeutic level.
p-value was calculated by Student t-test, Pearson chi-square (a) or Fisher-exact test (b);
*statistical significance (p-value<0.05); MAP: Mean arterial pressure; GFR: Glomerular filtration rate; DM: Diabetes mellitus
p-value was calculated by linear or logistic regression analysis; *statistical significance (p-value<0.05); There was some missing data at 2 hours after loading
BW: Body weight; p-value was calculated by logistic regression analysis; *statistical significance (p-value<0.05)
ICU: Intensive Care Unit; NICU: Neonatal Intensive Care Unit; p-value was calculated by Pearson chi-square or Fisher-exact test; statistical significance (p-value<0.05)