The urinary bladder cancer is the ninth most frequent cancer worldwide. Urothelial carcinoma is the most frequent histological type (>90%), about 70-80% of patients are diagnosed with non-invasive and low-grade tumours worldwide [1]. In Egypt, the National Population-Based Registry Program (2008-2011) reported that bladder cancer represented 6.94% of cancers in both sexes and about eighth of males’ cancers [2].
Glypican 3 is an oncofetal protein, that binds to the cell membrane through glycosylphosphatidylinositol anchors, having a role in regulating cell division and apoptosis by interacting with numerous growth factors [3]. Most of the studies investigated the neoplastic role of GPC3 focused on differentiating Hepatocellular Carcinoma (HCC) from its benign mimic lesions [4].
In HCC, GPC3 stimulates canonical Wnt signaling and enhances in-vitro and in-vivo tumoural growth. Thus, overexpression of GPC3 reflects an alternative mechanism in which Wnt activity is stimulated in HCC [3]. The same mechanism is supposed to have a potential role in urothelial carcinogenesis, but it is still debatable whether it may be caused by somatic mutations of members of the Wnt or Adenomatous Polyposis Coli (APC) or β-catenin (β-cat) signaling pathways. Different studies reported a relation between Wnt pathway and urothelial tumourigenesis, evidenced by loss of heterozygosity at the APC locus with no APC mutations identified in these tumours, up-regulation of β-cat by immunohistochemistry, CpG hypermethylation silencing of the region encoding Wnt inhibitory factor 1, or epigenetic silencing of the four secreted frizzled receptor proteins (antagonists of the Wnt signaling pathway). Also, other studies detected alteration of Wnt family members in up to 73% of 131 chemotherapy naive, muscle invasive, high-grade bladder tumours and that urothelial cancer associated one ribonucleic acid (which activates Wnt signaling) increases the cisplatin resistance of bladder cancer cells in-vitro [5].
Thus, the aim of this study was to evaluate the immunohistochemical expression of GPC3 marker in cases of UC to investigate the potential of GPC3 overexpression as a therapeutic target and correlate between GPC3 immunohistochemical expressions with available clinicopathological features of such tumours.
Materials and Methods
This is a retrospective study which included 125 cases of UC (90 cases of high-grade UC treated by radical cystectomies and 35 cases of low-grade UC treated by TURT) obtained through collection of archived paraffin blocks of UC, from the Pathology Department, Faculty of Medicine, Cairo University during the period from January 2016 till November 2017. Inclusion criteria; cases diagnosed as UC in the urinary bladder and the exclusion criteria; UC in other sites, UC in situ, other types of bladder cancer and cases with deficient clinical data. The medical records were revised and clinical data as age, gender, site, the histological type and grade of UC. The study took the approval of ethical committee in the faculty of Medicine, Cairo University (N-80-2016).
Each paraffin block was cut by rotator microtome at five microns thickness then mounted on glass slides to be stained by Haematoxylin and Eosin (H&E) for histopathological re-evaluation.
Histopathologic examination of H&E stained slides was performed under low power then high power for:
Confirmation of diagnosis and histological type of UC according to WHO classification of tumours of urothelial tract 2016 [6].
Revision of histological tumour staging and grading according to AJCC cancer staging 2017 [7].
Immunohistochemistry
Paraffin blocks were cut by rotator microtome at 5 μ thickness, mounted on charged slides and stained manually for immunohistochemistry. The sections were deparaffinised in xylene, for 10 minutes, then dehydrated in descending series of ethanol (100%, 96%, 70%), followed by washes in TBS (0.05 mmol/L Tris-buffer physiological saline, pH 7.4-7.6), for five minutes.
Antigen retrieval was achieved by heating the samples without boiling in 10 mmol/L sodium citrate buffer, pH 6.0 (200 mL) in a microwave oven. This treatment was conducted twice for seven minutes. The sections were washed in TBS buffer for 30 minutes.
The endogenous peroxide was blocked by 0.3% hydrogen peroxide in methanol for five minutes. The sections were washed in TBS for 15 minutes. To inhibit non-specific background staining; the samples were incubated in a superblock for 5-10 minutes at room temperature.
The primary antibody was monoclonal mouse GPC3 antibody (1G12 clone) purchased from Biocare Medical Company, 0.1 mL concentrated from the stock. The dilution was based on dilution experiments. The antibody was diluted with 20 mmol/L TBS, pH 7.4 (10 mmol/L CaCl2, 0.1% NaN3 and 1% BSA). The sections were incubated in the diluted antibody. The incubation took place in incubation boxes overnight.
The secondary antibody (4.5 μL biotinylated anti-mouse antibody in 1 mL of 1% BSA) was pipetted onto the sections and incubated in the moist box for 30 minutes. The secondary antibody was washed in TBS buffer for 15 minutes.
The final staining was done in Diaminobenzidine Tetrahydrochloride (DAB) solution (49 mL TBS-buffer, 34 mg imidazole, 17 μL 30% hydrogen peroxide and 1 mL 30% DAB), for 5-15 minutes. The slides were washed with distilled water, 70% ethanol for one minute, then in distilled water. The nuclei were stained with Mayer’s hematoxylin for 30 seconds as a counterstain. The extra stain was washed with tap water. The slides were then transferred through ascending ethanol series, and xylene before mounting.
Evaluation of Expression of GPC3
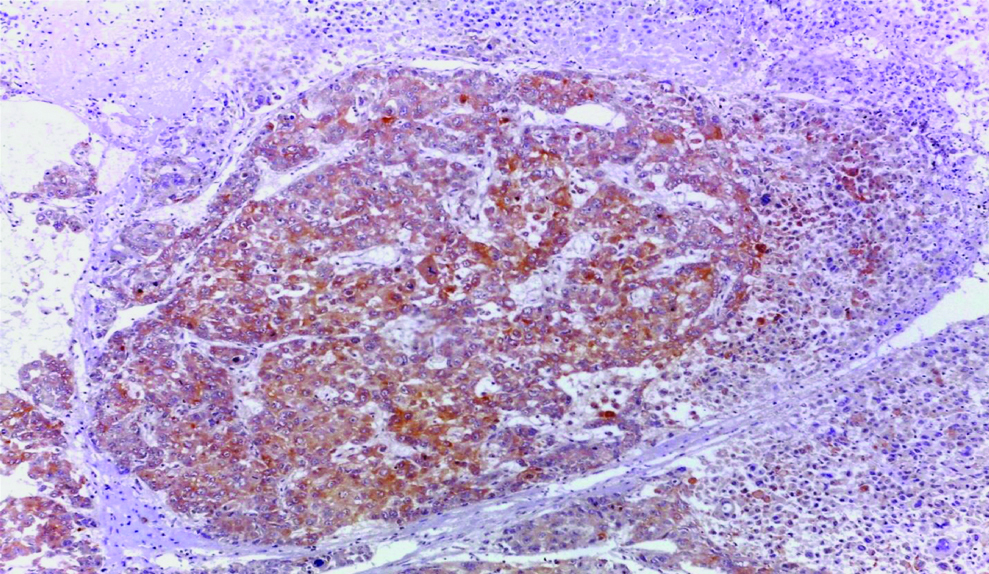
Tumour tissue sections were examined and scored under LEICA ICC50HD microscope at low power and then high power magnification. Cytoplasmic and/or membranous staining for GPC3 was accepted as positive. The control was HCC tissue as external control as shown in [Table/Fig-1]. Each slide was evaluated according to staining extent only. The extent of staining was scored semiquantitatively and was evaluated according to the percentage of stained cells. GPC3 was considered positive when >10% of tumour cells showed membranous and/or cytoplasmic staining, regardless of the intensity of staining. The results of GPC3 immunostaining in tumour cells were correlated with multiple prognostic factors (such as age, sex, site of the tumour, and histopathologic WHO grade).
Hepatocellular carcinoma (positive control) showing cytoplasmic GPC3 expression (original magnification X 100).
Statistical Analysis
Microsoft Excel 2016 was used for data entry and the Statistical Package for Social Science (SPSS version 25) was used for data analysis.
Simple descriptive statistics (arithmetic mean and standard deviation) was used for the summary of quantitative data and frequencies used for qualitative data.
The bivariate relationship was displayed in cross-tabulations and comparison of proportions was performed using the chi-square test.
The T-independent test was used to compare normally distributed quantitative data.
All p-values were two-sided. The p-values <0.05 were considered significant.
Results
Among the 125 cases of UC, 90 cases were selected as high-grade and 35 cases were low-grade [Table/Fig-2]. The mean age of all studied cases was 58.95 years at the time of pathological diagnosis. As regards, the gender, 105 cases out of 125 cases (84%) were males while females represented 20 cases (16%). The other clinicopathological variables are shown in [Table/Fig-3].
Non-invasive low grade papillary urothelial carcinoma H&E (original magnification X100)
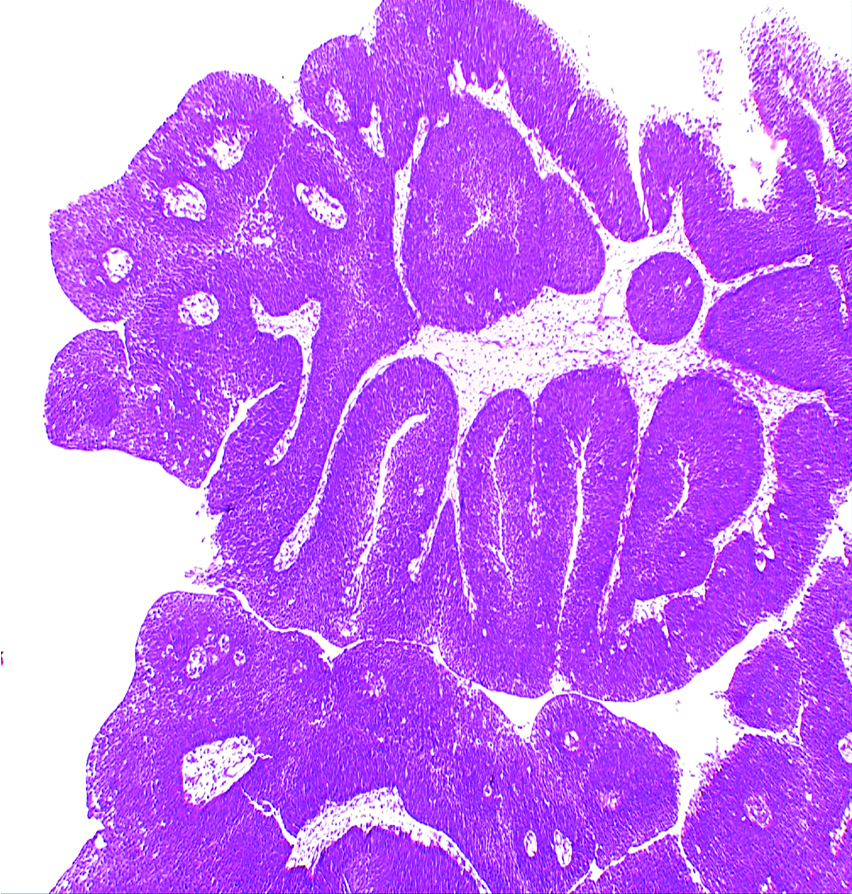
Clinicopathological characteristics of the studied cases of UC and its correlation with GPC3 expression.
| Parameter | Number (%) (n=125, 100%) | Negative GPC3 Expression (n=107, 85.6%) | Positive GPC3 Expression (n=18, 14.4%) | p-value |
|---|
| Age (mean, ±SD) (58.95, ±10.529) |
| <59 Years | 44 (35.2%) | 38 (35.5%) | 6 (33.3%) | 0.857 |
| ≥59 Years | 81 (64.8%) | 69 (64.5%) | 12 (66.7%) |
| Gender | | | | |
| Male | 105 (84%) | 88 (82.2%) | 17 (94.4%) | 0.191 |
| Female | 20 (16%) | 19 (17.8%) | 1 (5.6%) |
| Tumour site | | | | |
| Dome | 29 (23.2%) | 25 (23.4%) | 4 (22.2%) | 0.465 |
| Anterior wall | 15 (12%) | 13 (12.2%) | 2 (11.1%) |
| Posterior wall | 33 (26.4%) | 25 (23.4%) | 8 (44.4%) |
| Right lateral wall | 19 (15.2%) | 18 (16.8%) | 1 (5.6%) |
| Left lateral wall | 13 (10.4%) | 12 (11.2%) | 1 (5.6%) |
| Bladder neck | 6 (4.8%) | 6 (5.6%) | 0 (0%) |
| Whole cavity | 10 (8%) | 8 (7.4%) | 2 (11.1%) |
| Tumour size (mean, ±SD) (4.07, ±2.006) |
| <4 cm | 55 (44%) | 46 (43%) | 9 (50%) | 0.579 |
| ≥4 cm | 70 (56%) | 61 (57%) | 9 (50%) |
| Tumour gross appearance | (n=90) | (n=74) | (n=16) | |
| Fungating | 54 (60%) | 44 (59.5%) | 10 (62.5%) | 0.876 |
| Ulcerative | 32 (35.6%) | 27 (36.5%) | 5 (31.25%) |
| Infiltrating | 4 (4.4%) | 3 (4%) | 1 (6.25%) |
| Tumour histological subtypes | | | | |
| Classic UC | 36 (28.8%) | 29 (27.1%) | 7 (38.9%) | 0.690 |
| Papillary UC | 43 (34.4%) | 39 (36.4%) | 4 (22.2%) |
| UC with squamoid differentiation | 29 (23.2%) | 25 (23.4%) | 4 (22.2%) |
| UC with glandular differentiation | 9 (7.2%) | 6 (5.6%) | 3 (16.7%) |
| Sarcomatoid UC | 2 (1.6%) | 2 (1.9%) | 0 (0%) |
| Plasmacytoid UC | 1 (0.8%) | 1 (0.9%) | 0 (0%) |
| Clear cell UC | 1 (0.8%) | 1 (0.9%) | 0 (0%) |
| Micropapillary UC | 2 (1.6%) | 2 (1.9%) | 0 (0%) |
| Poorly differentiated UC | 2 (1.6%) | 2 (1.9%) | 0 (0%) |
| Tumour grade | | | | |
| Low grade | 35 (28%) | 33 (30.8%) | 2 (11.1%) | 0.085 |
| High grade | 90 (72%) | 74 (69.2%) | 16 (88.9%) |
| Tumour invasion | | | | |
| Invasive | 102 (81.6%) | 86 (80.4%) | 16 (88.9%) | 0.388 |
| Non-invasive | 23 (18.4% | 21 (19.6%) | 2 (11.1%) |
| Associated urinary bladder bilharziasis | | | | |
| Present | 29 (23.2%) | 26 (24.3%) | 3 (16.7%) | 0.478 |
| Absent | 96 (76.8%) | 81 (75.7%) | 15 (83.3%) |
| Tumour multifocality | | | | |
| Present | 9 (7.2%) | 8 (7.5%) | 1 (5.6%) | 0.770 |
| Absent | 116 (92.8%) | 99 (92.5%) | 17 (94.4%) |
| Tumour (T) stage | | | | |
| Ta | 22 (17.6%) | 20 (18.7%) | 2 (11.1%) | 0.047 |
| T1 | 14 (11.2%) | 13 (12.1%) | 1 (5.6%) |
| T2a | 3 (2.4%) | 3 (2.8%) | 0 (0%) |
| T2b | 18 (14.4%) | 15 (14%) | 3 (16.7%) |
| T3a | 14 (11.2%) | 8 (7.5%) | 6 (33.3%) |
| T3b | 43 (34.4%) | 37 (34.6%) | 6 (33.3%) |
| T4a | 11 (8.8%) | 11 (10.3%) | 0 (0%) |
| Lymph node (N) stage | (n=90) | (n=74) | (n=16) | |
| Nx | 6 (6.7%) | 6 (8.1%) | 0 (0%) | 0.423 |
| N0 | 49 (54.4%) | 37 (50%) | 12 (75%) |
| N1 | 16 (17.8%) | 14 (18.9%) | 2 (12.5%) |
| N2 | 18 (20%) | 16 (21.6%) | 2 (12.5%) |
| N3 | 1 (1.1%) | 1 (1.4%) | 0 (0%) |
| Lymphovascular emboli | (n=90) | (n=74) | (n=16) | |
| Present | 37 (41.1%) | 32 (43.2%) | 5 (31.25%) | 0.377 |
| Absent | 53 (58.9%) | 42 (56.8%) | 11 (68.75%) |
| Perineural invasion | (n=90) | (n=74) | (n=16) | |
| Present | 24 (26.7%) | 20 (27%) | 4 (25%) | 0.868 |
| Absent | 66 (73.3%) | 54 (73%) | 12 (75%) |
As regards, GPC3 expression in the current study, it was considered positive when >10% of tumour cells showed membranous and/or cytoplasmic staining, regardless of the intensity of staining. From the totally collected UC cases, 18 cases (14.4%) exhibited positive expression, while 107 cases (85.6%) exhibited negative expression. Two of the positive cases were low-grade UC and 16 were high-grade UC, suggesting that GPC3 has a higher expression in high-grade UC. Normal adjacent urothelium was detected in three cases, showing negative GPC3 expression, indicating a probability that GPC3 could be only expressed in the neoplastic tissue, an assumption which needs further investigation. Although there are different subtypes of UC, 7 of the 18 positive cases (38.8%) were of the classic subtype.
There was a statistically significant correlation between GPC3 expression and tumour (T) stage (p-value=0.047). However, no statistically significant correlation was detected between GPC3 expression and any of age, sex, tumour histological subtype, site, gross appearance, size, grade, invasion, multifocality, associated urinary bladder bilharziasis, lymphovascular emboli, perineural invasion and lymph node stage. The relationship between GPC3 expression and the clinicopathological variables are shown in [Table/Fig-3].
Discussion
In this study, the mean age of all selected cases was 58.95±10.529 years, ranging from 20 to 80 years, which was close to what was reported by El-Sharkawi F et al., and Mokhtar N et al., where their patients’ mean age was 61.3 and 67.5 years respectively [8,9].
The included cases showed male predominance, where 84% were males and 16% were females, with male/female ratio as 5.25:1, which was close to what was reported by El-Sharkawi F et al., and Mokhtar N et al., in their studies about bladder cancer in Egypt, where 74.3% and 76.5% were males, while 25.7% and 23.5% were females, respectively [8,9].
GPC3 expression in UC in this study was positive in 14.4% (18/125) and negative in 85.6% (107/125), which was similar to what was observed by Aydin O et al., Gailey MP et al., Baumhoer D et al., Xylinas E et al., where their positive cases were (35.2%; 38/108), (12.2%; 6/49), (16%; 7/43), and (6%; 19/311) respectively [3,4,10,11].
Normal adjacent urothelial tissue was detected in only three UC cases in this study, showing negative GPC3 expression [Table/Fig-4], in accordance with results reported by Baumhoer D et al., and Al-Saraireh Y et al., in their studies, indicating that GPC3 antibody can be used to differentiate between neoplastic and non-neoplastic urothelial lesions [10,12].
Normal urothelium showing negative GPC3 expression (original magnification X400).
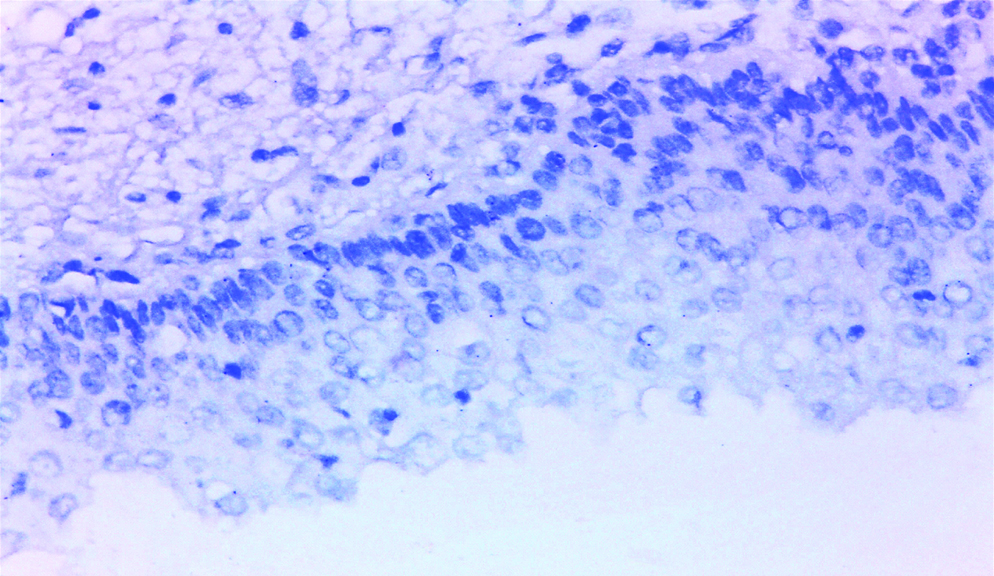
No significant statistical relation was detected between patients’ age and GPC3 expression (p-value=0.857), which was close to what was reported by Xylinas E et al., where p-value was 0.44 [11].
According to gender, 17 males and 1 female showed positive GPC3 expression, while 88 males and 19 females showed negative GPC3 expression, with non-significant statistical relation between sex and GPC3 expression (p-value=0.191), which was in agreement with what was stated by Xylinas E et al., 2014, where males were more predominant in both positive and negative cases for GPC3 expression and p-value was 0.77 [11].
The collected cases in the present study showed UC ranged in size between 1 to 10 cm in maximum dimension, with mean size 4.07 cm; 56% were ≥4 cm and 44% were <4 cm. These results were close to what Gondo T et al., had reported, where 68.3% were ≥3 cm and 31.7% were <3 cm [13].
The most common site in the urinary bladder for UC in the collected cases was the posterior wall (26.4%), followed by dome (23.2%), then right lateral wall (15.2%), anterior wall (12%), left lateral wall (10.4%), whole cavity (8%) and bladder neck (4.8%), which was discordant with the results of Palou J et al., reporting that existence of UC at multiple locations in the urinary bladder was the most common (27.7%), followed by left lateral wall (24.1%), then right lateral wall (23.5%), trigone (10.7%), posterior wall (10.2%), unknown site (2.6%), dome (1.1%), anterior wall (0.9%) and bladder neck (0.6%) [14].
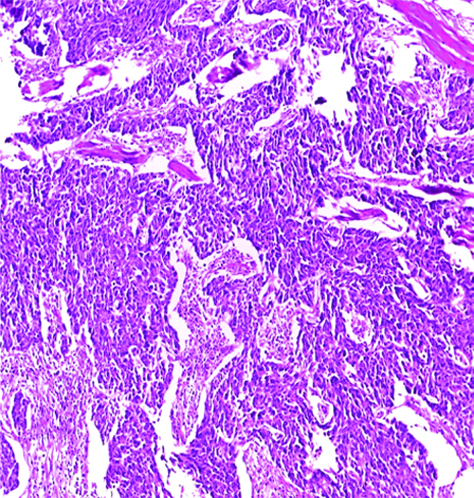
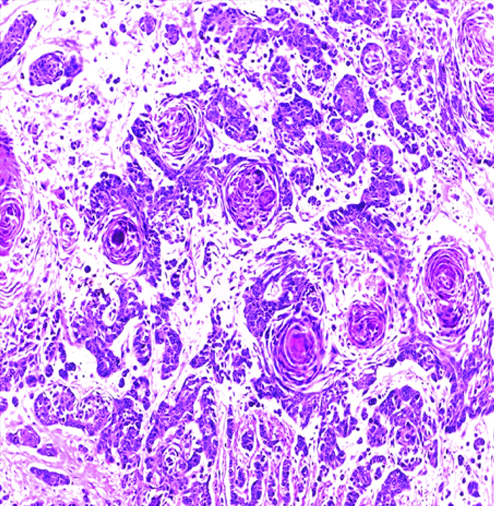
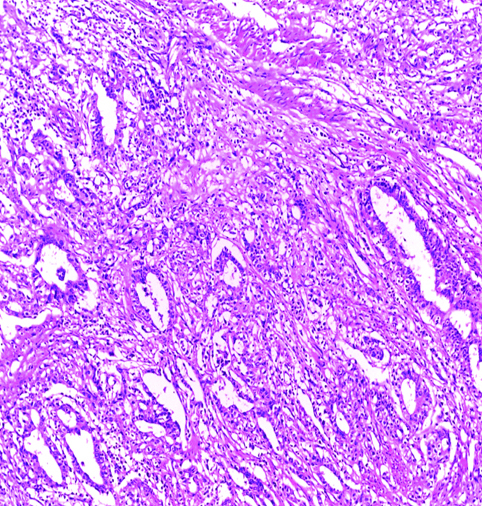
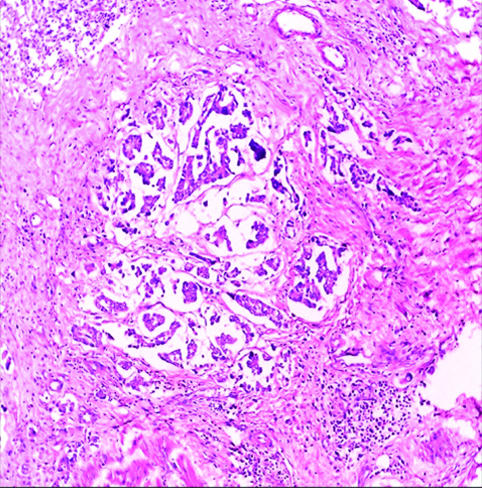
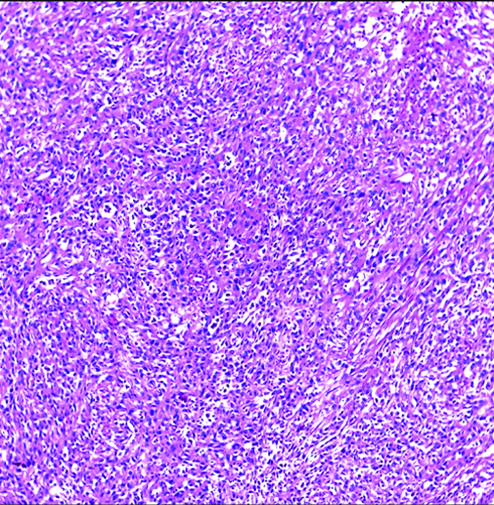
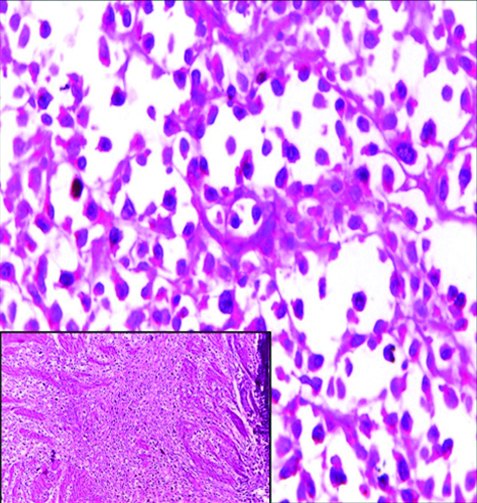
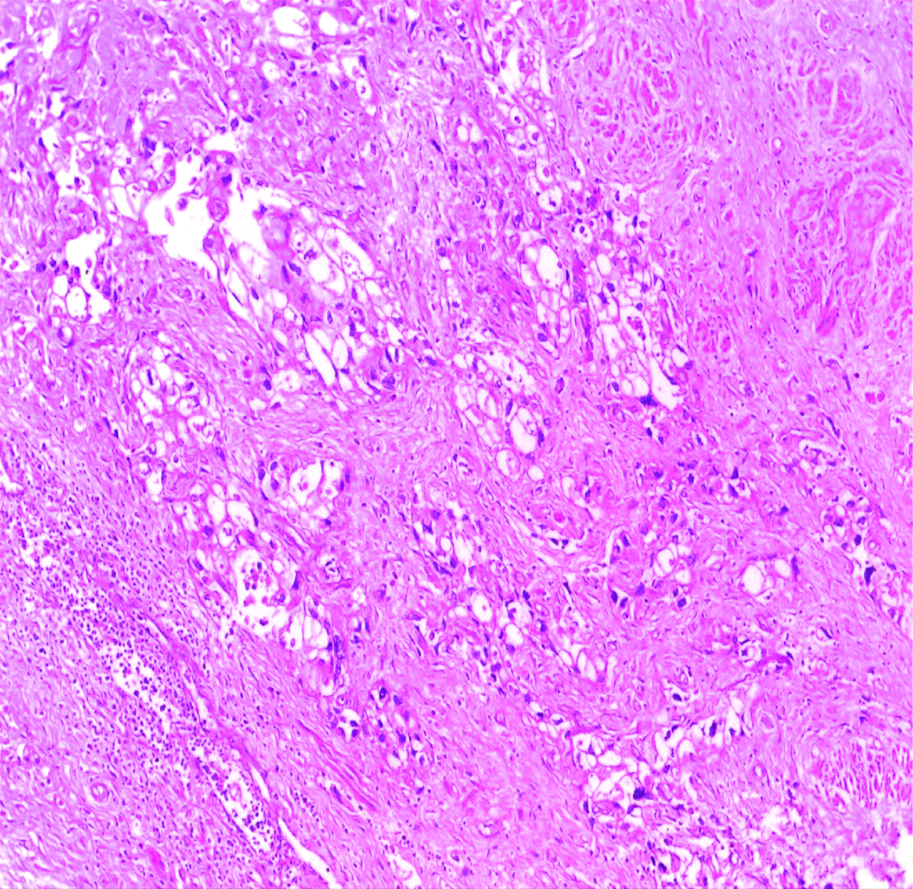
The most common gross appearance for UC in the selected cases was the fungating pattern (60%), while ulcerative and infiltrative patterns were (25.6%) and (4.4%) respectively. Papillary UC was the most common histological type in the selected cases (34.4%) [Table/Fig-5], classic UC (28.8%) [Table/Fig-6], UC with squamoid differentiation (23.2%) [Table/Fig-7], UC with glandular differentiation (7.2%) [Table/Fig-8], sarcomatoid UC [Table/Fig-9], micropapillary UC [Table/Fig-10], poorly differentiated UC [Table/Fig-11] (1.6% each), plasmacytoid UC [Table/Fig-12] and clear cell UC [Table/Fig-13] (0.8% each), in contrast to the results of Xylinas E et al., who reported that conventional UC was the most common in his study (75.4%), UC with squamoid differentiation (11.4%), UC with glandular differentiation (3.8%), multiple variants (3.3%), sarcomatoid UC (2%), small cell UC (2%), micropapillary UC (1.7%), and plasmacytoid UC (0.4%) [15].
Invasive high-grade papillary urothelial carcinoma H&E (original magnification X100).
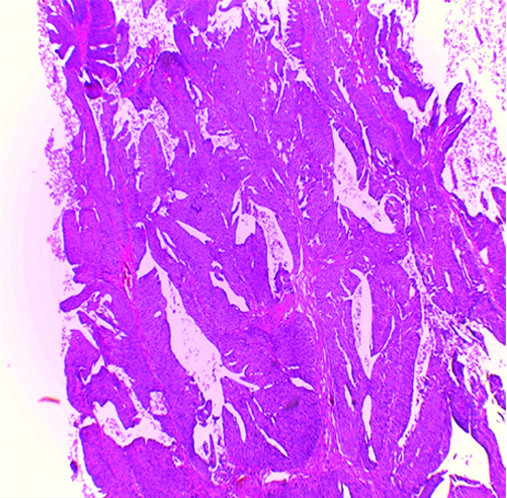
Invasive high-grade classic urothelial carcinoma H&E (original magnification X100).
Invasive high-grade urothelial carcinoma with squamoid differentiation H&E (original magnification X100).
Invasive high-grade urothelial carcinoma with glandular differentiation H&E (original magnification X100).
Invasive high-grade sarcomatoid urothelial carcinoma H&E (original magnification X100).
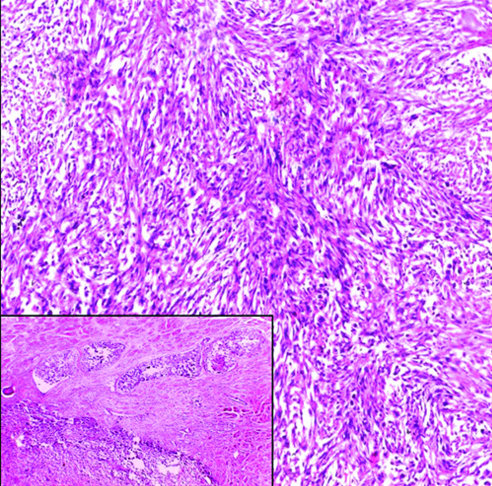
Invasive high-grade micropapillary urothelial carcinoma H&E (original magnification X100).
Invasive high-grade poorly differentiated urothelial carcinoma H&E (original magnification X100).
Invasive high-grade plasmacytoid urothelial carcinoma H&E (original magnification X100).
Invasive high-grade urothelial carcinoma, clear cell variant H&E (original magnification X100).
In the present work, the tumour size, site in urinary bladder, gross appearance, and histological type had insignificant correlation with GPC3 expression (p-value=0.579, 0.465, 0.876, 0.69 respectively). These findings were not thoroughly evaluated by other comparative studies.
Only 29 cases in this study showed urinary bladder bilharzial infestation. Nine cases showed tumour multifocality. In radical cystectomy cases, 37 cases showed lymphovascular emboli, while 24 cases showed perineural invasion.
No significant correlation was found between the selected cases regarding urinary bladder bilharzial infestation, tumour multifocality, lymphovascular emboli [Table/Fig-14], perineural invasion [Table/Fig-15] and GPC3 expression (p-value=0.478, 0.77, 0.377, 0.868 respectively). These findings also were not thoroughly evaluated by other comparative studies.
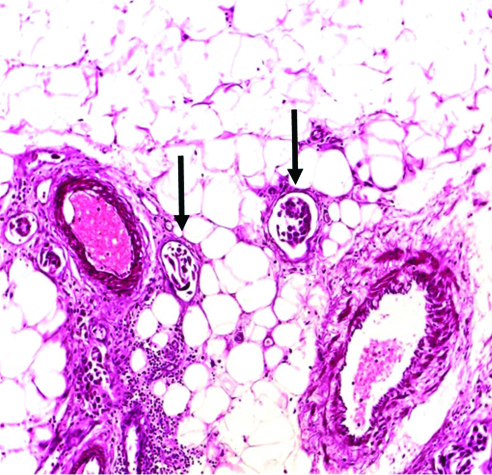
Vascular emboli (arrows) in invasive high-grade urothelial carcinoma H&E (original magnification X100).
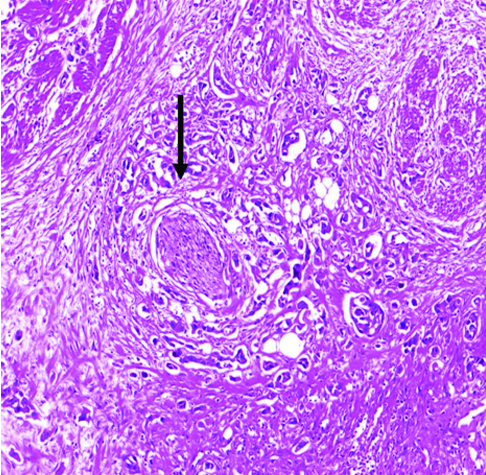
Perineural invasion (arrow) in invasive high-grade urothelial carcinoma H&E (original magnification X100).
In this study, most of the cases showing positive GPC3 expression were high-grade UC (16/18) [Table/Fig-16,17], while the last two positive cases were low-grade cases (2/18) [Table/Fig-18,19,20 and 21], signifying that GPC3 may be helpful in differentiating between low- and high-grade UC cases, which needs further investigations.
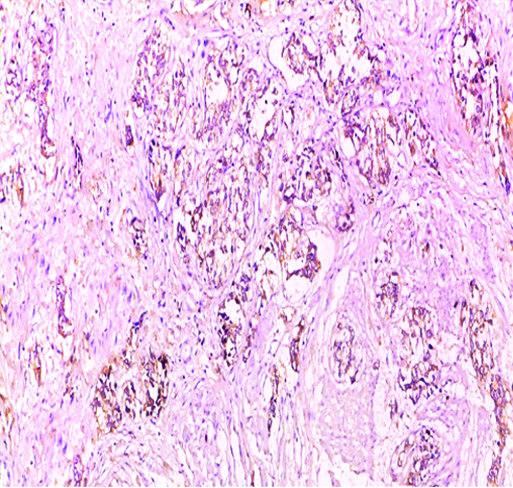
Invasive high-grade urothelial carcinoma showing positive cytoplasmic and focal membranous GPC3 expression (original magnification X 100).
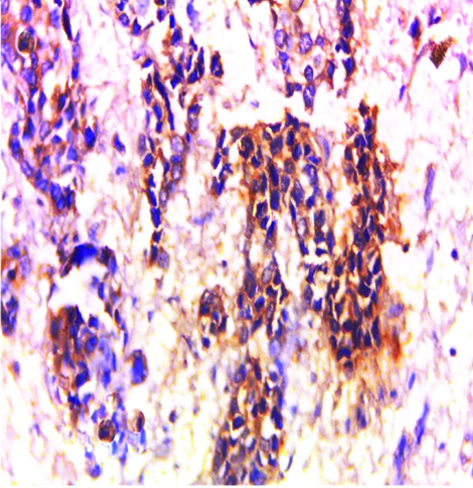
Invasive high-grade urothelial carcinoma showing positive cytoplasmic GPC3 expression (original magnification X 400).
Low-grade papillary urothelial carcinoma showing focal positive cytoplasmic GPC3 expression (original magnification X 100).
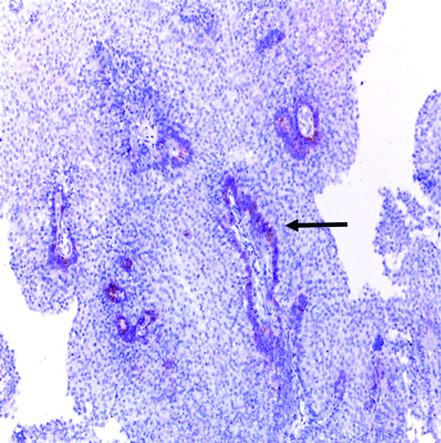
Low-grade papillary urothelial carcinoma showing positive cytoplasmic GPC3 expression (original magnification X 400).
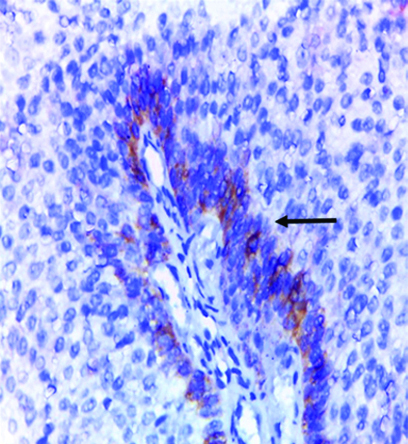
Low-grade papillary urothelial carcinoma showing focal positive membranous and cytoplasmic GPC3 expression (original magnification x 100).
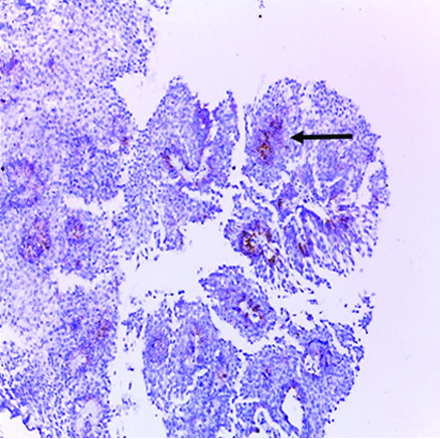
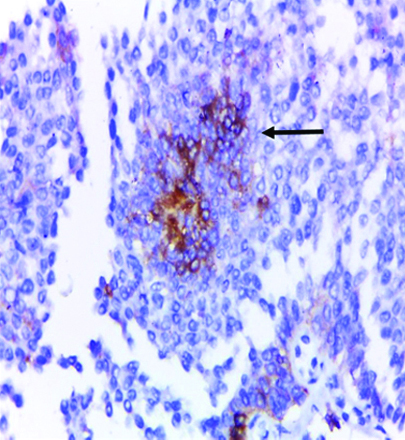
Low-grade papillary urothelial carcinoma showing focal positive membranous and cytoplasmic GPC3 expression (original magnification X 400). (Arrows: GPC3 expression in basal urothelial cells only)
No significant correlation was detected between tumour grade and GPC3 expression (p-value=0.085). These findings were similar to that observed by Xylinas E et al., 2014, where no significant correlation was detected between tumour grade and GPC3 (p-value=0.12) [11], in contrast to what reported by Aydin O et al., 2015, where their results showed significant correlation, with p-value=0.003 [3].
In the present study, the expression of GPC3 was detected only in the basal urothelial cells in low-grade positive cases and does not reach the superficial (umbrella) cells [Table/Fig-18,19,20 and 21], while in high-grade positive cases; the expression of GPC3 was diffuse. This finding was not previously noted in other comparative studies and needs to be investigated in future studies.
Most of cases in this study (81.6%) showed vesical mural invasion and the rest (18.4%) were non-invasive; no significant correlation was detected between tumour invasion and GPC3 expression (p-value=0.388), which was in agreement with Aydin et al., 2015, who also reported that no significant relation detected between tumour invasion and GPC3 expression (p-value=0.386) [3].
Most UC cases enrolled in this study presents with T3b stage (34.4%), while Ta was 17.6%, T1 was 11.2%, T2a was 2.4%, T2b was 14.4%, T3a was 11.2% and T4a was 8.8%, in disagreement with Mokhtar N et al., 2016, where T2 stage was the most predominant stage [9].
A significant correlation was detected between T stage and GPC3 expression (p-value=0.047), which was discordant with the results of Xylinas E et al., reporting that no significant correlation was detected between T stage and GPC3 expression (p-value=0.12) [11].
In radical cystectomy cases collected in this study, the lymph node N stage in most of the cases was N0 (54.4%), Nx was 6.7%, N1 was 17.8%, N2 was 20% and N3 was 1.1%. No significant correlation was detected between N stage and GPC3 expression (p-value=0.423). No data reported about lymph node status by other comparative studies.
Several studies had suggested different mechanisms for the pathogenesis of UC such as mutation of Harvey Rat Sarcoma (HRAS), Fibroblast Growth Factor Receptor 3 (FGFR3), Phosphoinositide 3-Kinase (PI3K), P53, Phosphatase and Tensin Homologue (PTEN) and Retinoblastoma (RB) oncogenes or dysregulation of Wnt signaling pathway. Because of these several mechanisms, urothelial carcinogenesis may occur by any or all of them, which could explain that the majority of UC cases in this study showed negative GPC3 expression, where carcinogenesis could be happened by a different mechanism other than stimulation of Wnt pathway.
Limitation
The limitation of this study was the small number of the collected sample due to high cost of GPC3, recommending a larger sample in future studies and the lack of reliable registry of the patient and follow-up, so the survival rate could not be assumed in this study.
Conclusion
To sum up, in this study, there was a significant correlation between the expression of GPC3 and tumour (T) stage. GPC3 is a novel and worthwhile therapeutic target for cancer. Future studies should be carried out on larger scale in correlation with other factors as survival rate and therapeutic effect for proper understanding of GPC3 potential role in urothelial carcinogenesis, in attempt to develop novel therapeutic strategy targeting GPC3 antigen and also for more investigations of its possible role in differentiation between neoplastic and non-neoplastic urothelial tissue, as well as between low and high-grade UC.