Partial Hypopituitarism Following Miscarriage: A Rare Presentation
Monisha Priyadarshini Kumar1, Nairmeen Haller2, Daniela Ciltea3
1 Department of Internal Medicine, Cleveland Clinic Akron General, Akron, OH, USA.
2 Department of Research, Cleveland Clinic Akron General, Akron, OH, USA.
3 Department of Internal Medicine, Cleveland Clinic Akron General, Akron, OH, USA.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Monisha Priyadarshini Kumar, 1 Akron General Avenue, Akron, Ohio, USA.
E-mail: dr.mpdk@gmail.com
Hypopituitarism is a rare disorder with a prevalence of 45.5 cases per 100,000 individuals. The term “Selective or Partial Hypopituitarism” refers to the loss of at least one, but not all pituitary hormones. Gravid women are at high risk of developing hypopituitarism due to significant increase in the size of pituitary gland during pregnancy, and thus high propensity to undergo pituitary necrosis following hypotension from any source including labour, miscarriage or major blood loss. Although, it is an uncommon condition, early recognition and management play a crucial role in patient care. Here, a rare case of a 23-year-old Caucasian female who developed partial hypopituitarism, predominantly central hypothyroidism and minor gonadal hormonal deficiencies due to haemorrhage, during an episode of miscarriage is reported. She was managed medically with appropriate hormone replacement therapy with clinical improvement in symptoms.
Central hypothyroidism, Pregnancy, Selective hypopituitarism
Case Report
A 23-year-old Caucasian female presented with chief complaint of worsening fatigue for a period of 2-3 months which initially started as mild tiredness during work and progressed to the point of significant exhaustion leading to quit her current job and be homebound. Patient also had a medical history of Systemic Lupus Erythematosus (SLE), diagnosed as a teenager which was treated and eventually went into remission. At the time of admission, patient was not on any medications for SLE and reported that she was not following-up with a rheumatologist for the same.
Five months prior to current presentation, she had a hospital admission for profuse vaginal bleeding at 19 weeks of gestation and was diagnosed with a miscarriage. She subsequently underwent dilatation and curettage, and was found to be hypotensive, as well as anaemic with haemoglobin of 10 g/dL (12.0-15.5 g/dL). She was given intravenous fluids, iron supplementation, and was discharged home post-procedure. Since then, she has been amenorrhoeic, but did not seek medical care. In addition, she developed progressive fatigue, limiting her daily activities, depressed mood, and had 10 kg unintentional weight loss over a course of 2-3 months. At this point, she presented again to the hospital for further evaluation. Her vital signs on admission includes blood pressure of 104/72 mmHg, heart rate 62 per minute, and she remained afebrile. On physical examination, she had a flat affect, cachectic and malnourished appearance. She denied symptoms of joint pain, joint swelling or rash.
Laboratory workup revealed haemoglobin of 9.9 g/dL (12.0-15.5 g/dL), normal white blood cell count, electrolytes and glucose. We also checked erythrocyte sedimentation rate and C-reactive protein titres to evaluate for lupus flare given her medical history, however they were within normal limits, ruling out any active inflammatory process. Given excessive fatigue, thyroid function testing was performed and resulted in Thyroid Stimulating Hormone (TSH) level of 0.097 uIU/mL (0.35-3.74 uIU/mL), free thyroxine (T4) 0.72 ng/dL (0.76-1.46 ng/dL), and free triiodothyronine (T3) 1.1pg/mL (2.2-4 pg/mL). Given inappropriately low TSH in the setting of low free T4 and free T3, she was diagnosed with secondary hypothyroidism, and Magnetic Resonance Imaging (MRI) of the brain was ordered for further evaluation of pituitary and hypothalamus.
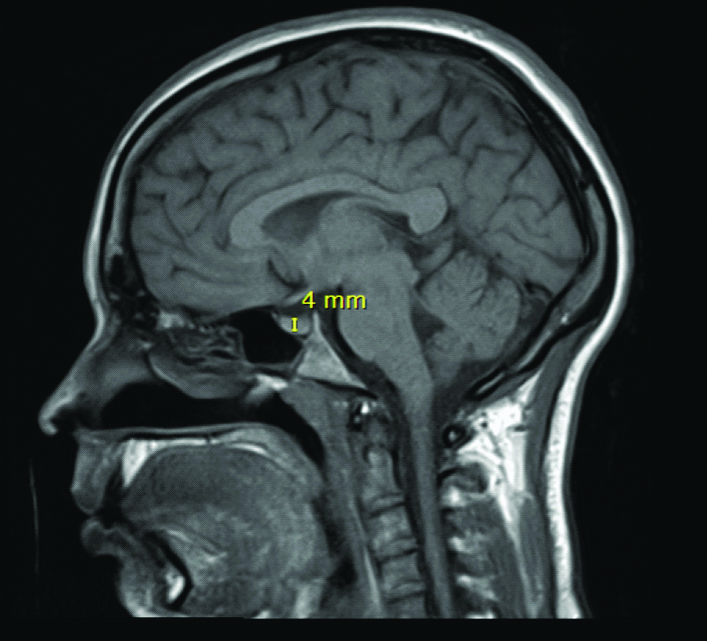
Workup for the central cause of hypothyroidism revealed a hypoplastic pituitary gland (4 mm) on MRI Brain with and without contrast [Table/Fig-1]. Further testing was done to screen for other pituitary hormone deficiency which resulted in prolactin 2.8 ng/mL (2.8-29.2 ng/mL), Follicle Stimulating Hormone (FSH) 4.36 mIU/mL (low normal), Luteinising Hormone (LH) 2.61 mIU/mL (low normal), Insulin-like growth factor-1 243 ng/mL (119-328 ng/mL), and Adrenocorticotropic Hormone (ACTH) 44 pg/mL (8-42 pg/mL). Hence, it was concluded that the patient developed hypoplastic hypopituitarism from possible infarction secondary to the episode of blood loss and hypotension during her recent miscarriage.
T1-weighted MRI of the brain, Sagittal section, showing pituitary gland measuring 4 mm.
She was started on levothyroxine and was discharged on day seven in stable condition and with improvement in symptoms. Also, she was scheduled for an outpatient follow-up in two weeks to monitor clinical response and possible gonadal hormone replacement therapy. Dismally, the patient did not report back.
Discussion
In adults, the size of the pituitary gland normally measures 10 mm in length, 5-10 mm in depth and 10-15 mm in width. Women in their childbearing years have larger glands. Autopsy studies [1,2] have revealed the presence of enlarged pituitary glands in pregnant women. In-vivo [3] studies have shown that during pregnancy, the pituitary gland can increase in size up to 136% compared to the non-pregnant female control with 45% increment occurring during the first trimester [4]. The size of the pituitary gland before pregnancy normally measures 9.6-10 mm in height which increase to 10.2-12 mm in immediate postpartum period and returns to original size within six months [5].
The growth of the pituitary gland is primarily due to hyperplasia of lactotrophs and forming pregnancy cells. Pregnancy cells are modified prolactin-producing cells that arise from lactotrophs. These changes are mainly due to an increase in estrogen and progesterone, and these cells are responsible for prolactin secretion during pregnancy, which is important in preparing the breast tissue for lactation [6]. The above-mentioned increase in size of the pituitary gland during gestation occurs without any concomitant increase in blood supply. In addition, there is significant compression of blood vessels in an enlarging pituitary. Overall, all these factors make the pituitary susceptible to ischaemia [5].
The most prevalent risk factors that can cause compromised blood supply during pregnancy are severe postpartum haemorrhage causing hypotensive shock and disseminated intravascular coagulation [7]. The compromise in blood supply leads to necrosis of the enlarged pituitary causing loss of glandular functions and leading to hypopituitarism. This phenomenon of post-partum ischaemic pituitary necrosis is identified as Sheehan’s Syndrome. This condition is commonly identified by failure of lactation in the post-partum female due to prolactin deficiency, which discursively helps in early identification of hypopituitarism [8].
Unlike the postpartum hypopituitarism, which is relatively common, the patient described in the present case report had miscarriage during the 19th week of her pregnancy leading to hypotensive shock, which compromised the blood supply to the pituitary subsequently leading to necrosis and hypopituitarism. The prevalence of miscarriage or spontaneous abortion remains high ranging anywhere between 8-20% prior to 20th week of gestation [9]. It is believed that the actual prevalence might be higher since many women have early miscarriages prior to realising that they are pregnant [9]. There are multiple risk factors for miscarriage such as older age, alcohol use, smoking, teratogenic medication use, substance abuse, and trauma [10].
The clinical presentation of hypopituitarism mainly depends on rapidity of disease development and severity of hormone deficiency [11]. Anterior pituitary hormones including ACTH, TSH, FSH, LH, prolactin and Growth Hormone (GH) are commonly affected. Postpartum women often present with the complaint of lack of lactation, which leads to the testing of these hormones and eventually to diagnosis of hypopituitarism. However, the patient described in our case, did not complete her pregnancy and hence did not have a similar presentation. She presented with fatigue as her chief complaint, which led to the diagnosis of hypothyroidism and was subsequently classified as central hypothyroidism. In addition, the patient had amenorrhoea and the further evaluation for the same led to the diagnosis of gonadotropin deficiency [11]. GH is less commonly affected and its deficiency manifests with non-specific symptoms leading to poor quality of life, decrease in lean body mass and decline in bone mineral density. Deficiency of ACTH can lead to secondary Adrenal Insufficiency (AI) and subsequent cortisol deficiency, which can present as hypotension, hyponatraemia, or hypoglycaemia. Early identification of AI and corticosteroid replacement is crucial to prevent mortality in such patients. Replacement of other hormones as required by clinical scenario need to be considered [11,12] Our patient did not develop ACTH or GH deficiency, but primarily had hypothyroidism and gonadotropin deficiency, hence the diagnosis of partial hypopituitarism was established which was treated with necessary hormone replacement. There are multiple reports of hypopituitarism due to Sheehan’s syndrome following pregnancy [13-16] but our case is unique that the hypopituitarism developed following a miscarriage.
Conclusion
In summary, this report describes an uncommon case of partial hypopituitarism after an episode of miscarriage in a young female with the chief presenting complaint as fatigue. Hypopituitarism in pregnancy remains a rare condition where early recognition and management plays a crucial role in patient care. Often, a lack of lactation is reported by postpartum women leading to early diagnosis of hypopituitarism. However, these symptoms are not present in women following miscarriage, which complicates the diagnosis. Recognition of signs and symptoms of hypopituitarism along with detailed review of history is crucial in timely diagnosis and management of this rare phenomenon in women following miscarriage.
[1]. Scheithauer BW, Sano T, Kovacs KT, Young WF, Ryan N, Randall RV, The pituitary gland in pregnancy: a clinicopathologic and immunohistochemical study of 69 casesMayo Clinic Proceedings 1990 65:461-74.10.1016/S0025-6196(12)60946-X [Google Scholar] [CrossRef]
[2]. Goluboff LG, Ezrin C, Effect of pregnancy on the somatotroph and the prolactin cell of the human adenohypophysisThe Journal of Clinical Endocrinology & Metabolism 1969 29:1533-38.10.1210/jcem-29-12-15334186795 [Google Scholar] [CrossRef] [PubMed]
[3]. Gonzalez J, Elizondo G, Saldivar D, Nanez H, Todd L, Villarreal J, Pituitary gland growth during normal pregnancy: an in vivo study using magnetic resonance imagingThe American Journal of Medicine 1988 85:217-20.10.1016/S0002-9343(88)80346-2 [Google Scholar] [CrossRef]
[4]. Karaca Z, Tanriverdi F, Unluhizarci K, Kelestimur F, Pregnancy and pituitary disordersEuropean Journal of Endocrinology 2010 162:116710.1530/EJE-09-0923e [Google Scholar] [CrossRef]
[5]. Laway B, Mir S, Pregnancy and pituitary disorders: Challenges in diagnosis and managementIndian Journal of Endocrinology and Metabolism 2013 17:99610.4103/2230-8210.12260824381874 [Google Scholar] [CrossRef] [PubMed]
[6]. Levin G, Rottenstreich A, Prolactin, prolactin disorders, and dopamine agonists during pregnancyHormones 2018 doi:10.1007/s42000-018-0071-z10.1007/s42000-018-0071-z30341577 [Google Scholar] [CrossRef] [PubMed]
[7]. Diri H, Karaca Z, Tanriverdi F, Unluhizarci K, Kelestimur F, Sheehan’s syndrome: new insights into an old diseaseEndocrine 2015 51:22-31.10.1007/s12020-015-0726-326323346 [Google Scholar] [CrossRef] [PubMed]
[8]. Kilicli F, Dokmetas HS, Acibucu F, Sheehan’s syndromeGynecological Endocrinology 2012 29(4):292-95.10.3109/09513590.2012.75245423245206 [Google Scholar] [CrossRef] [PubMed]
[9]. Dugas C, Whitten R, Miscarriage 2018 Treasure Island (FL)StatPearls Publishing [Google Scholar]
[10]. Feodor Nilsson S, Andersen PK, Strandberg-Larsen K, Nybo Andersen AM, Risk factors for miscarriage from a prevention perspective: a nationwide follow-up studyBJOG 2014 121(11):1375-84.10.1111/1471-0528.1269424548778 [Google Scholar] [CrossRef] [PubMed]
[11]. Higham CE, Johannsson G, Shalet SM, HypopituitarismLancet 2016 388(10058):2403-15.10.1016/S0140-6736(16)30053-8 [Google Scholar] [CrossRef]
[12]. Kim SY, Diagnosis and treatment of hypopituitarismEndocrinology and Metabolism 2015 30(4):443-55.10.3803/EnM.2015.30.4.44326790380 [Google Scholar] [CrossRef] [PubMed]
[13]. Matsuzaki S, Endo M, Ueda Y, Mimura K, Kakigano A, Egawa-Takata T, A case of acute Sheehan’s syndrome and literature review: a rare but life-threatening complication of postpartum hemorrhageBMC Pregnancy and Childbirth 2017 17(1):18810.1186/s12884-017-1380-y28615049 [Google Scholar] [CrossRef] [PubMed]
[14]. Sert M, Tetiker T, Kirim S, Kocak M, Clinical report of 28 patients with sheehans syndromeEndocrine Journal 2003 50:297-301.10.1507/endocrj.50.29712940458 [Google Scholar] [CrossRef] [PubMed]
[15]. Al-Tubaikh JA, Sheehan syndrome (postpartum hypopituitarism)Internal Medicine 2010 :247-48.doi:10.1007/978-3-642-03709-2_4310.1007/978-3-642-03709-2_43 [Google Scholar] [CrossRef]
[16]. Baskaran DD, Sheehan’s syndrome: a case reportJournal of Medical Science and Clinical Research 2017 :5doi:10.18535/jmscr/v5i10.6310.18535/jmscr/v5i10.63 [Google Scholar] [CrossRef]