Propofol, a di-isopropylphenol, is a popular intravenous anaesthetic induction agent, mainly due to its short duration of action that allow for rapid emergence combined with minimal residual sedation. However, a well recognised disadvantage of its use is pain on injection [1] with an incidence of pain in up to 36% of cases [2]; the exact mechanism by which propofol causes pain has not been fully elucidated. The most accepted theory suggests that when propofol comes into contact with the vascular endothelium it causes release of mediators from the kinin cascade which results in a characteristic pattern of pain beginning at the site of injection and slowly spreading proximally along the course of the vessel carrying the propofol solution [3]. A recent study claims that non-selective ligand-gated cation channels such as Transient Receptor Potential (TRP) Ankyrin 1 (TRPA1) and TRP Vanilloid 1 (TRPV1) are the predominant molecular entities mediating activation of peripheral nerve endings by general anaesthetics [4]. The most popular method for reducing the incidence of pain is to add a small amount of lignocaine to propofol with or without tourniquet [5].
Propofol MCT/LCT is a new formulation of propofol emulsion with similar pharmacokinetics and efficacy as standard propofol, but reduces the amount of free propofol in the emulsion thereby causing less pain on injection than propofol [5,6]. An emulsion containing long and medium-chain triglycerides (Propofol-Lipuro®) reduces the incidence of pain on injection from 14.7 to 2.7% without lignocaine [7].
Etomidate is a well known substituted imidazole induction agent that shares most of the beneficial characteristics of propofol (e.g., rapid onset/offset and minimal residual sedation) and is also associated with a very high degree of haemodynamic stability [8]. The hyperosmolality of the propylene glycol formulation may cause direct injury to vascular endothelium resulting in local physical damage and release of histamine into circulation [9].
Although etomidate causes adreno-cortical suppression, a single injection to induce anaesthesia will only produce a transient and clinically insignificant interference with adrenocortical function [10]. Etomidate is now available as a lipid emulsion of etomidate-MCT/LCT which is associated with significantly less pain on injection [10].
Materials and Methods
After permission from the Institutional Ethics Committee (Letter no. MMC 72, dated 15.01.16, issued from Midnapore Medical College, West Bengal) 65 surgical patients of American Society of Anaesthesiologists (ASA) physical status I and II, aged between 18 to 60 years posted for general surgical procedures under General Endotracheal Anaesthesia (GETA) with operative duration of less than two hours were chosen for this prospective double blind study in a government medical college, from March 2016 to August 2017.
Patients allergic to study drugs, having severe co-morbid conditions including adrenocortical insufficiency, having history of intake of any analgesic drugs anytime in the preoperative period, having any anatomical deformity in upper limb, and having communication difficulties were excluded from this study. Patients were also excluded from the study if it took more than one attempt to cannulate a vein.
This study was unique in that all patients received in one arm propofol-MCT/LCT and simultaneously in the other arm received etomidate-MCT/LCT, while the pain on injection was evaluated. Thus, the subjective variation of pain on injection was totally eliminated as the patient was his or her own comparator regarding which formulation causes more pain.
We randomly selected 65 patients (i.e., total study arm is 130) for this study. This was based on a previous study based on reduction from 30% of pain on injection and setting alpha and beta values at 0.05 and 95% respectively [6]. The total number of hands for injection was estimated at 110 i.e., a minimum of 55 patient arms for i.v. injection. We added around 18% more to compensate for any loss of power resulting from any dropouts.
Patients were advised to keep six hours of fasting to carbohydrate based solid food and take sips of water till two hours before surgery. All patients were randomised by opening an opaque envelope inside the operating room containing the computer generated random assignment number ranging from 1 to 65.
On arrival of patient to operating room two 20 G i.v. cannula were inserted at the dorsum of each hand after ECG, NIBP and pulse oximeter monitoring were attached. No analgesic drugs were given before induction. Each i.v. cannula was flushed with 10 mL of normal saline over 5 seconds to confirm that the patient did not have any pain before the study drug was injected.
Each subject received 5 mL of propofol-MCT/LCT i.v. through either right or left arm and simultaneously 5 mL of etomidate-MCT/LCT through the other arm as follows-subjects with an even identification number received etomidate-MCT/LCT in left dorsum of hand and propofol-MCT/LCT in right dorsum of hand, while patients with odd identification number received etomidate-MCT/LCT in right dorsum of hand and propofol-MCT/LCT in left dorsum of hand. The drugs were prepared by similar 5 cc syringes by anaesthesiologists not involved in the study. As the drugs are similar in appearance (milky white) the patients and observers were also suitably blinded for the study.
Both the drugs were given manually by two different anaesthesiologists simultaneously while an assistant kept time with a stopwatch. The two study drugs were given simultaneously in the two arms of every patient and @1mL/sec through the top ports of the 20 G i.v. cannula such that 5 mL of study drugs were completed in 5 seconds.
The incidence of injection pain during injection of the two drugs was assessed by a four-point scale [6] (0- no pain,1- verbal complaint of pain, 2- withdrawal of the arm and 3- both verbal complaint and withdrawal of arm) by a separate observer who was in constant verbal communication with the patient and noted immediately after completion of injections.
Immediately after study i.v. agents (i.e., 5 mL of propofol-MCT/LCT and 5 mL of etomidate-MCT/LCT) were given the general anaesthesia induction was completed using sevoflurane at 5% concentration along with injection atracurium 0.5 mg/kg to facilitate intubation. The study ended when the endotracheal tube was placed and confirmed.
We also assessed haemodynamic variables in terms of systolic blood pressure, diastolic blood pressure, mean blood pressure and heart rate on arrival in OT (baseline), at the time of injection of study drugs, at the time of laryngoscopy, and at the time of endotracheal tube insertion. The study also assessed any rare side-effects like myoclonus, bronchospasm, allergic reaction etc., associated with the drugs.
Inj. fentanyl 1.5 μg/kg i.v. bolus and inj. aqueous diclofenac 75 mg/mL, 1 mL in running normal saline bottle, were given to maintain analgesia. We maintained anaesthesia with sevoflurane at 1 to 2% concentration and intermittent boluses of atracurium and reversed neuromuscular blockade with neostigmine 0.05 mg/kg with glycopyrrolate 0.01 mg/kg at the end of each surgery.
Statistical Analysis
Data were collected in study proforma and analysed by a biostatistician at the end of the study using Stata 14.0 software (windows version) of StataCorp LP, Texas 77845, USA. All numerical variables were found to be normally distributed by Kolmogorov-Smirnov goodness-of-fit test other than SpO2 values. Comparison of pain scores at the time of injections between the two studied drugs (paired comparison) were done by Student’s paired samples t test. The p-value <0.001 was regarded as statistically significant. To observe the statistical significance of change in individual haemodynamic variables over time we did repeated measures analysis of variance (ANOVA) followed by Tukey’s-test for post-hoc comparison between any two time points. Data were represented as mean±Standard Deviation (SD) or ratios.
Results
This prospective double blind randomised study was conducted in 65 consenting patients over 18 months who received 5 mL of propofol-MCT/LCT i.v. through either right or left arm and simultaneously 5 mL of etomidate-MCT/LCT through the other arm. [Table/Fig-1] shows the CONSORT flow chart of this study.
[Table/Fig-2] shows the demographic profile of the 65 patients in terms of age, height, weight, body mass index (BMI), gender, ASA grading and duration of surgery and anaesthesia.
Demographic variables of the 65 patients.
| Demographic Variables | Values (mean±SD or number) |
|---|
| Age (yrs) | 35.17±10.70 |
| Height (metre) | 1.54±0.08 |
| Weight (kg) | 56.40±6.08 |
| BMI (kg/m2) | 23.74±0.83 |
| Male/Female (number) | 37/28 |
| ASA grade I/II (number) | 50/15 |
| Duration of surgery (min.) | 84.94±20.56 |
| Duration of anaesthesia (min.) | 96.02±19.77 |
[Table/Fig-3] shows the incidence of injection pain of propofol-MCT/LCT and etomidate-MCT/LCT. Propofol administration was associated with pain (of any severity) in 26 out of 65 patients i.e., 40.0% (95% Confidence Interval (CI) 28.09-51.91%) whereas etomidate administration was associated with pain (of any severity) in 6 out of 65 patients i.e. 9.2% (95% CI 2.19-16.27%). Comparison of pain scores at the time of injections between the two studied drugs (paired comparison) was done using Student’s paired samples t-test. The incidence of injection pain of propofol-MCT/LCT was associated with significantly more pain compared to etomidate-MCT/LCT (p<0.001).
Incidence of pain on injection.
| Mean | SD | No. (percentage) | t | df | p-value |
|---|
| Pain propofol-MCT/LCT | 0.45 | 0.5871 | 26 (40%) | 5.92 | 64 | <0.001* |
| Pain etomidate-MCT/LCT | 0.09 | 0.2917 | 6 (9.2%) |
*Statistically significant, df-degree of freedom
We measured pain using a four point rating scale (from 0-no pain to 3-both verbal complaint and withdrawal of arm). Twenty three patients (35.4%) complained of injection pain of grade 1 severity and three patients (4.6%) complained of injection pain of grade 2 severity with propofol-MCT/LCT, where as six patients (9.2%) complained of injection pain of only grade 1 severity with etomidate-MCT/LCT. The distribution of injection pain according to gradation of pain is demonstrated in [Table/Fig-4].
Distribution of pain on injection according to gradation of pain.
| Pain Gradation | Propofol-MCT/LCT | Etomidate-MCT/LCT |
|---|
| Number of patients | Percentage (%) | Number of patients | Percentage (%) |
|---|
| 0 | 39 | 60 | 59 | 90.8 |
| 1 | 23 | 35.4 | 6 | 9.2 |
| 2 | 3 | 4.6 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 |
| Total | 65 | 100 | 65 | 100 |
In our study, incidence of injection pain was higher among females than males (53.57% vs 45.95%); in ASA II category than ASA I category (73.33% vs 42%); in left hand (non-dominant) compared to right hand (60.61% vs 18.75%).
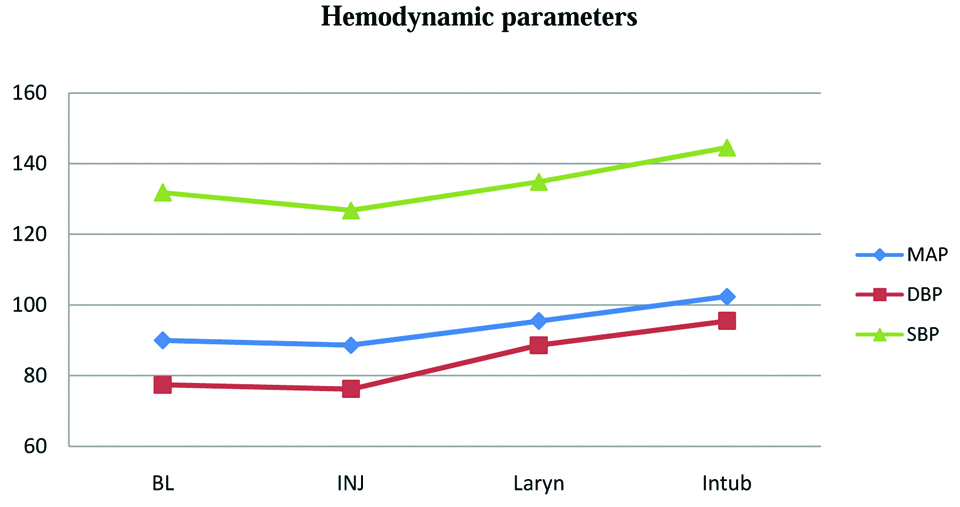
Comparison of Systolic (SBP), Diastolic (DBP) and Mean Blood Pressure (MAP) at Baseline (BL), time of Injection (INJ), at Laryngoscopy (Laryn) and Intubation (Intub) was done [Table/Fig-5]. ANOVA test followed by Tukey’s test for post hoc comparison showed significant increase in SBP, DBP and MAP at laryngoscopy and intubation compared to at time of drug injection.
Significant increase of haemodynamic parameters at larngoscopy and intubation.
Of the 65 patients, myoclonus was seen in 19 patients (29.23%). We did not encounter any other side effects such as bronchospasm, allergic reaction etc.
Discussion
This prospective double blind randomised study was intended to compare the incidence of injection pain between MCT/LCT preparations of propofol and etomidate. As pain is subjective and each person has an individual perception of pain, we compared the two drugs in two arms of the same patient simultaneously to avoid any subjective variation using a 4 point pain score.
Propofol administration was associated with pain (of any severity) in 26 out of 65 cases (40.0%) whereas etomidate administration was associated with pain (of any severity) in 6 out of 65 cases (9.2%). The mean pain score with propofol administration was significantly more than with etomidate administration (40% vs 9.2%; p-value<0.001).
The incidence of injection pain of propofol-MCT/LCT in this study is 40%. The only other available study by Kaur S et al., showed the incidence of injection pain of propofol-MCT/LCT is 26.7% [11]. This variation of pain may be due to different surgical study populations.
Pain on injection after giving etomidate-MCT/LCT preparations is highly variable across studies ranging from 0 to 63.2% [11-14]. Our incidence of pain on injection of etomidate-MCT/LCT is on the lower side (9.23%), which corresponds to studies of Nyman Y et al., and Sharma A et al., [12,14]. The higher incidence of pain in the few other studies confirms our initial assumption that pain is a subjective phenomenon, and there will always be a variation of incidence of pain across patients.
Only one study so far has compared MCT/LCT preparations of etomidate and propofol in 60 cardiac patients posted for non-cardiac surgeries [11]. Pain on injection occurred in 26.7% patients of propofol-MCT/LCT group as compared to 6.7% patients of etomidate-MCT/LCT group. The difference is statistically not significant. Compared to this study, our study showed that propofol-MCT/LCT preparation caused significantly more pain than etomidate-MCT/LCT (40% vs 9.2%) which was statistically significant (p-value <0.001). Apart from the different surgical population of these two studies the main difference is that our study avoids subjective variation, the other study does not do so.
One study till date have used 50% etomidate-MCT/LCT with 50% of propofol-LCT (not MCT/LCT) and found this preparation to be painless [13]. Propofol-MCT/LCT preparations cause less injection pain than propofol-LCT preparations [15], it can be assumed that 50% admixture of etomidate-MCT/LCT and propofol-MCT/LCT will be painless. However, this theoretical assumption requires further study for corroboration.
In our study, we have found significant increase in systolic, diastolic and mean blood pressure at laryngoscopy and intubation compared to time of study drug injection. This is an interesting finding and cannot be corroborated as no other study has till date assessed either SBP or DBP at the time of laryngoscopy after giving i.v. preparations of etomidate and propofol. Almost all studies focussed on intubation and immediate post-intubation haemodynamic variables as compared to baseline values [11,13,14]. However, most studies have found a decrease in systolic, diastolic and mean blood pressure at laryngoscopy and intubation compared to baseline. This discrepancy can be explained by some limitations of our study.
The study drug volume of 5 mL in two arms at induction was approximately half the complete induction dose for either propofol or etomidate-MCT/LCT preparations. For completion of general anaesthesia induction all patients required sevoflurane 5% by mask. This low dose of i.v. induction agents may have caused a higher surge of blood pressure at laryngoscopy and intubation in our study compared to a full induction dose of either propofol or etomidate.
Similarly, though 19 patients (29.23%) suffered from myoclonus this side-effect cannot be pointed out to either propofol-MCT/LCT or etomidate-MCT/LCT as all the patients have received both the drugs simultaneously. Other studies have found other side effects with the use of etomidate-MCT/LCT preparations like nausea, vomiting [14] and bradycardia, apnoea with the use of propofol-MCT/LCT preparations [11] but we have not encountered such events in this study.
Limitation
Our study suffered from some other limitations. We injected 5 mL of etomidate-MCT/LCT or propofol-MCT/LCT to each and every patient. As we have administered both the study drugs in same individual, side-effects cannot be pointed out to any specific drug. Similarly, the change in haemodynamic parameters in terms of SBP, DBP, MAP and heart rate with progression of time cannot be attributed to either of the drugs. We also cannot comment whether there will be more incidence or change in severity of pain on injection if any dose more than 5 mL of either etomidate-MCT/LCT or propofol-MCT/LCT is given intravenously. However, we have avoided the subjective bias of pain with simultaneous injection of two drugs at a similar rate to the same patient so that the information obtained is unique and very much relevant.
Conclusion
If the same patient was given similar volumes of MCT/LCT preparation of either etomidate or propofol, he/she will have much less pain on injection with etomidate compared to propofol, without significant side effects. Thus, the MCT/LCT preparation of etomidate may be chosen for intravenous induction of general anaesthesia, particularly for pain-sensitive and anxious individuals.
*Statistically significant, df-degree of freedom