GBC is a rare entity as a result of which there is no established standard treatment recommendation. In the absence of phase III trials, adjuvant treatment for GBC is based on retrospective studies on small cohort of patients. Despite all the possible treatment combinations of surgery with Chemotherapy (CT) and Radiotherapy (RT), 5-year overall survival for stage II and III remains 9-33% and 0-25% respectively [1-8]. Despite curative surgery, Loco-Regional Recurrence (LRR) is as high as 86% [9].
Adjuvant RT has a proven role in reducing loco-regional failure in several gastro-intestinal malignancies e.g., oesophagus, stomach, rectum, anal canal etc., [10-13]. Cisplatin is a radiosensitizing agent and has shown to improve the loco-regional control rate when given concurrently with RT in oesophageal cancer [10]. To the best of our knowledge, RT with concurrent Cisplatin (CDDP) has never been tried in GBC after curative cholecystectomy. Hence, the present study was designed to evaluate the effects of adjuvant RT with concurrent CDDP in patients of GBC with pathological stage II and III following curative cholecystectomy.
Materials and Methods
This was a retrospective study in which database of patients registered in our outpatient department of SS Hospital, Varanasi, India between January 2016 and January 2017 were evaluated. The patients of GBC who had up-front curative cholecystectomy followed by adjuvant RT with concurrent CDDP were selected. Patients who had neo-adjuvant CT were excluded from this study. Pathological stage II and III were included in the study. Patient profile, tumour characteristics, treatment that the patient had received, disease status and survival outcome were noted down from the database. Since this was a retrospective study, consent of patients and permission of Institutional Ethics Committee was not required.
All the selected patients had received external beam RT with concurrent CDDP at 35 mg/m2 once a week for a maximum of five cycles (range, 2-5). Computerised tomography based RT simulation was done and RT planning was done on eclipse treatment planning system. The patients were treated on linear accelerator (Varian Unique Performance) with 6 MV photon using 3 Dimensional Conformal Radiotherapy (3 DCRT) techniques to a dose of 45 Gy in 25 fractions over five weeks. Tumour bed along with regional lymph nodes was treated. OS and DFS were the primary end points. Survival was calculated from the date of surgery. The survival outcomes were measured using Kaplan-Meier method and log rank test was used to compare two parameters. SPSS 16.0 software was used for statistical analysis.
Results
We could identity 12 patients who could meet the selection criteria of our study. Eleven of these twelve patients were female. The median age was 52 years with a range of 24 to 70 years. Demographic profile of our patients is given in [Table/Fig-1]. All the patients had curative surgery except one where only part of gallbladder was resected. R0 resection was seen in nine patients while one had R1. Margin status was not known in two patients. None of these patients had neo-adjuvant chemotherapy. There were no major gastrointestinal or haematological toxicities which necessitated dose modification of chemotherapy or RT dose reduction. Mean RT dose and chemotherapy cycle was 45 Gy and 4 respectively. The mean RT duration was 34.6 days. Treatment compliance was good in all except in one patient who suffered from progression of disease during treatment. This odd patient had incomplete gallbladder removal and could receive only two cycles of concurrent CDDP and 41.4 Gy in 23 fractions [Table/Fig-2]. None of our patients had any major complication or late toxicity.
Tumour characteristics of the patients.
| Characteristic | Value |
|---|
| Histology |
| Adenocarcinoma | 11 |
| Adeno-squamous | 1 |
| Grade |
| I | 5 |
| II | 3 |
| III | 2 |
| Unknown | 2 |
| T stage |
| T2 | 7 |
| T3 | 5 |
| N stage |
| N0 | 5 |
| N1 | 3 |
| Nx | 4 |
| M stage |
| M0 | 12 |
| Group stage |
| II | 6 |
| IIIA | 3 |
| IIIB | 3 |
| Lympho-vascular invasion |
| Positive | 5 |
| Negative | 3 |
| Unknown | 4 |
| Perineural invasion |
| Positive | 4 |
| Negative | 4 |
| Unknown | 4 |
| Resection margin |
| Positive | 1 |
| Negative | 9 |
| Unknown | 2 |
Treatment characteristics.
| Characteristic | Value |
|---|
| Surgery |
| Open cholecystectomy | 10 |
| Laparoscopic cholecystectomy | 2 |
| RT dose |
| 41.4 Gy | 1 |
| 45.0 Gy | 10 |
| 50.0 Gy | 1 |
| RT duration |
| <36 days | 11 |
| >36 days | 1 |
| Chemotherapy |
| 2 cycles | 1 |
| 4 cycles | 2 |
| 5 cycles | 9 |
Data was analysed at the end of June’ 2018. The overall median follow-up period was 20.4 months and 23.5 months for those who were alive. At the time of analysis, one patient had died due to the disease, one was Lost To Follow-Up (LFU) without disease, one was LFU with disease and the rests were alive. Two patients had distant failure; one had loco-regional failure while one had local failure.
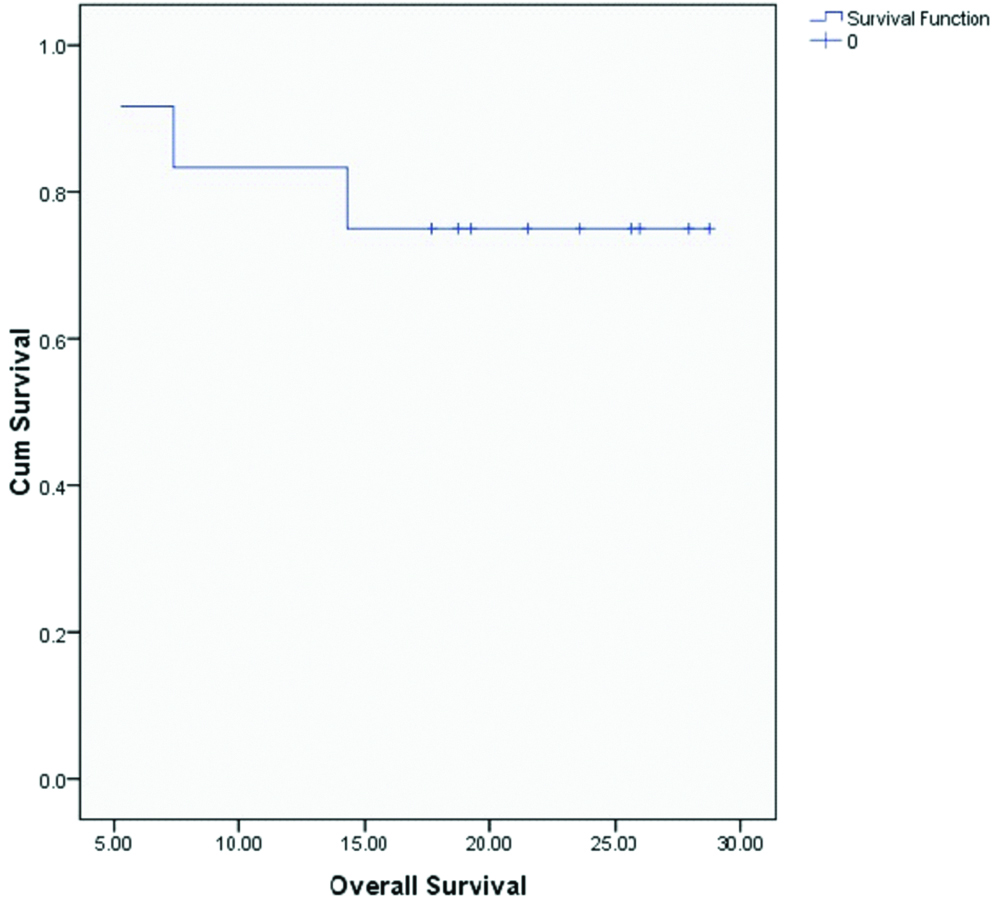
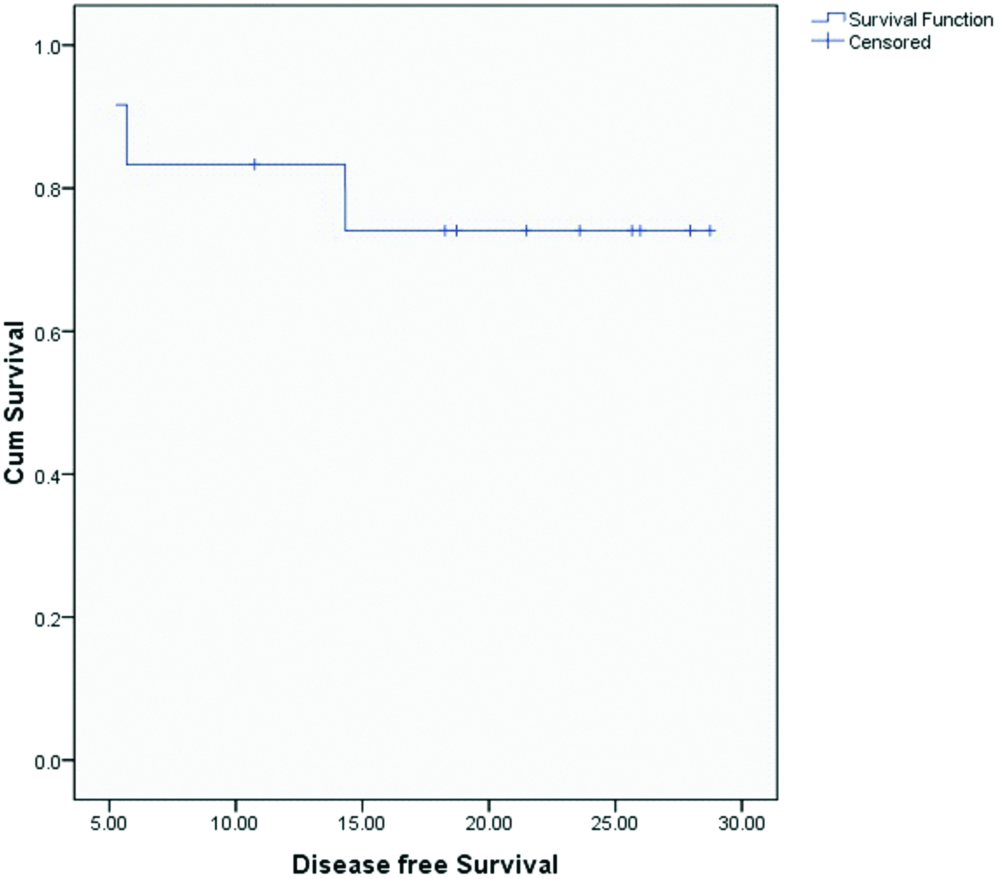
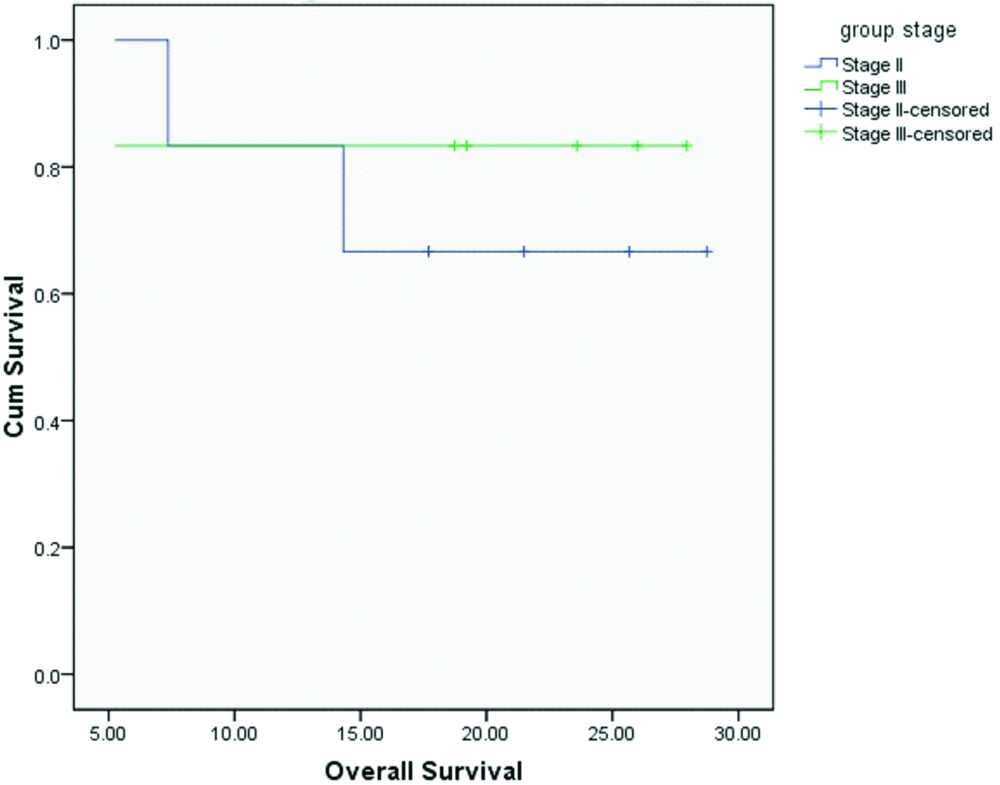
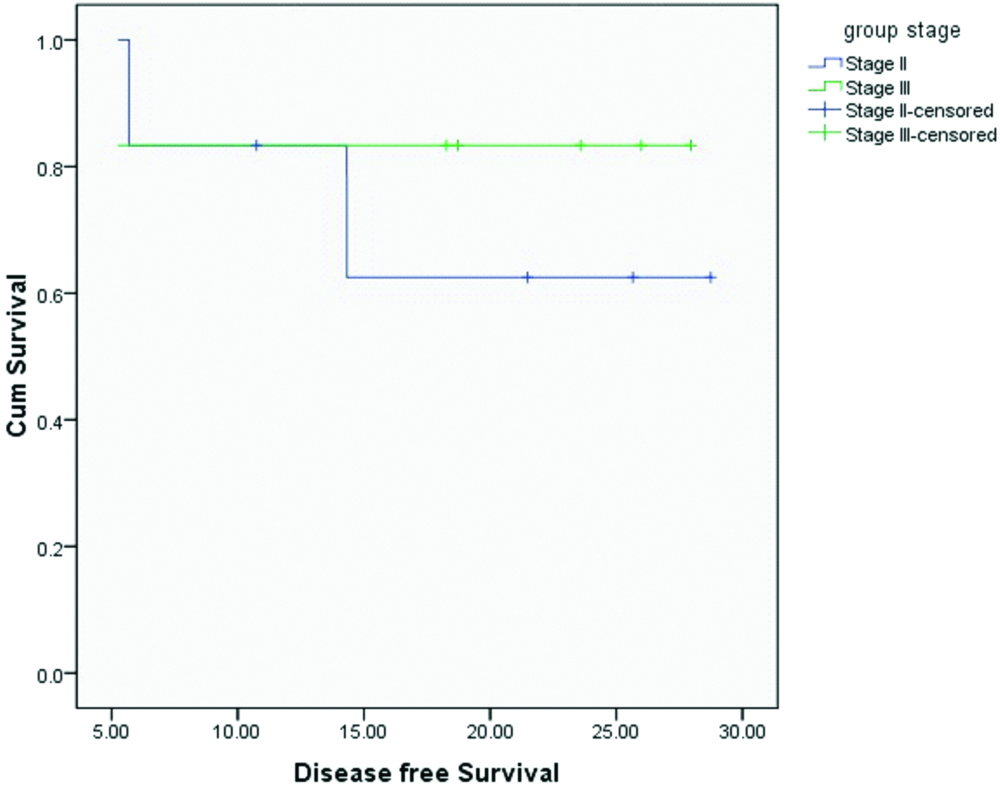
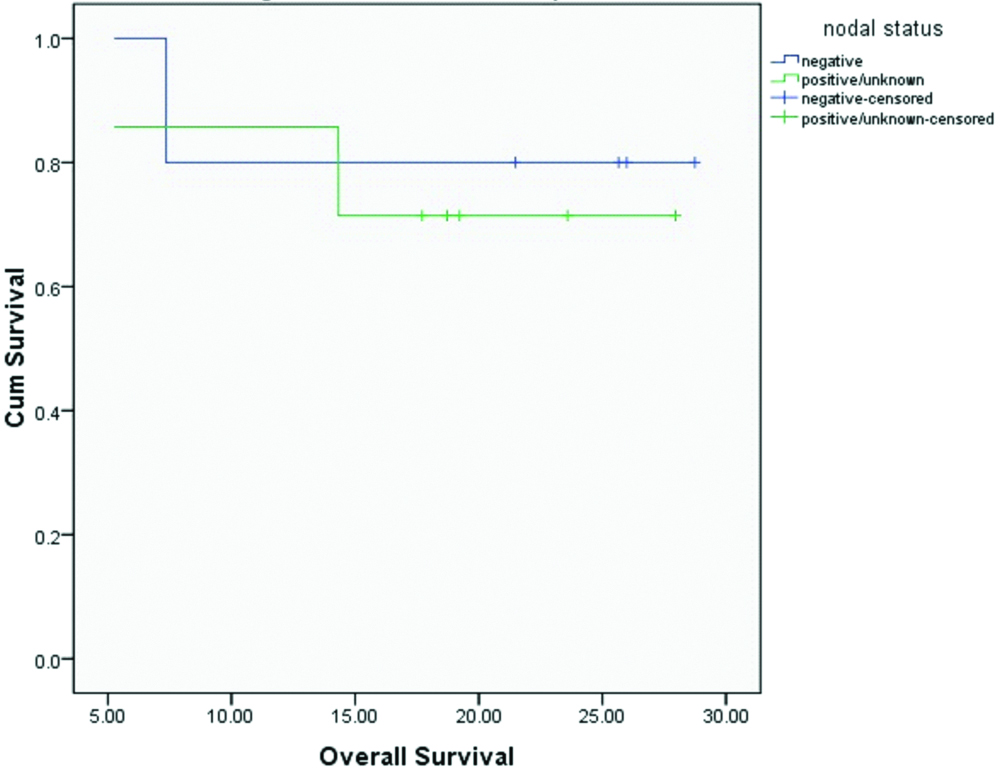
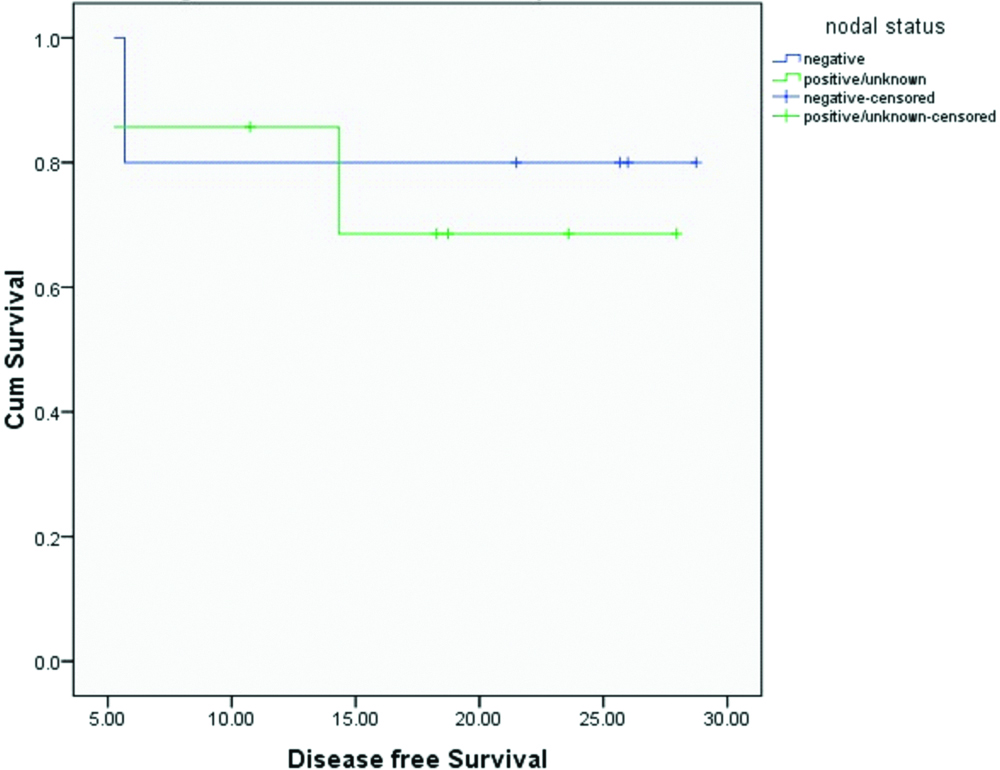
Median OS and DFS for the entire cohort were 20.4 and 20.1 months respectively. OS and DFS at 2-years for the entire cohort was 75% and 72% respectively [Table/Fig-3,4]. Since there was only one patient with R1 resection, it was not appropriate to do subset analysis based on margin status. On subset analysis OS and DFS at two years for both stage II and III had no significant difference (p=0.0599 and 0.554 respectively; [Table/Fig-5,6]). On univariate analysis OS and DFS for node positive and node negative diseases were not significantly different (p=0.751, p=0.713 respectively; [Table/Fig-7,8]).
Overall survival for all patients.
Disease free survival for all patients.
Overall survival based on stage.
Disease free survival based on stage.
Overall survival based on nodal status.
Disease free survival based on nodal status.
Discussion
The study was done to evaluate the role of concurrent CDDP with RT in stage II and III GBC patients after they had undergone curative cholecystectomy.
Extended cholecystectomy is the only well established treatment for GB cancer stage II and III [1-3,14,15]. However, less than 10% of patients undergo such radical procedure [10,16,17]. Only two of our patients had extended cholecystectomy while the rest had simple cholecystectomy.
Despite curative surgery, GBC has the propensity of very high loco-regional and distant failure. Study conducted at Memorial Sloan-Kettering had reported loco-regional failure in 45% of their patients [18]. In our series of patients we observed one local and one loco-regional failure. The patient who failed locally had partial gallbladder removal and had poor prognostic features i.e., positive Lympho-Vascular Invasion (LVI), Peri-Neural Invasion (PNI), and resection margin. Due to poor compliance only two cycles of concurrent chemotherapy was possible and RT was stopped after 41.4 Gy. The other patient who had LR failure had positive PNI as the only high risk factor. One patient who had laparoscopic cholecystectomy failed at the port site. Salvage surgery followed by RT with electron was done. She was disease free at the time of analysis. The patient who had distant failure had stage IIIB disease with LVI and PNI positive features.
Loco-regional failure is the most common cause of death in patients of GBC where curative surgery was not followed by adjuvant RT [9,19]. RT has a well established role in enhancing the loco-regional control in several gastro-intestinal malignancies [10-13]. Several studies have been conducted to prove its efficacy in GBC. Unfortunately, due to very low incidence of GBC there are only retrospective and small prospective cohort studies to show the advantage of adding RT to surgery [14-19].
Bosset JF et al., was one of the first to conduct a trial where patients who had curative cholecystectomy for GBC were given adjuvant RT to a dose of 54 Gy in 30 fractions [20]. At the time of analysis 5 out of 7 patients were alive with no evidence of disease. They recommended the use of adjuvant RT after curative surgery for GBC.
Kothari N et al., in their retrospective study of 73 patients of GBC had reported the survival benefit of adjuvant RT after curative surgery over surgery alone [21]. The median OS for the combination arm was 48.4 months while the same for surgery alone arm was 22.3 months.
Yang L et al., had compared the outcome of 84 patients who had radical cholecystectomy for GBC with 43 cases who had adjuvant RT as well [22]. The OS at 5 years were 7.2% and 1.9% which was statistically significant in favour of adjuvant RT arm.
Adjuvant Concurrent Chemo-Radiation (CCRT) has a proven advantage over RT alone in several gastro-intestinal malignancies. Addition of 5-FU based chemotherapy to RT in adjuvant setting in GBC has been explored in several retrospective studies with promising results in few and disappointing in the rests.
A retrospective study was carried out by Kresl JJ et al., on 21 GBC patients [23]. Maximum safe resection was attempted in these patients. One had stage I disease, eight had stage III disease while rest had stage IV disease. Subsequent to surgery they had received two cycles of 5-FU or 5-FU and Leucovorin based chemotherapy concomitant with RT. Median RT dose was 54 Gy (range 50.4-60.8 Gy). Median survival for the entire cohort was 2.6 years. Due to severe acute toxicity second cycle of chemotherapy could not be given in six patients. In addition, dose reduction had to be done in three patients. Late toxicities were observed in two patients.
Czito BG et al., had conducted a retrospective study in 22 patients of non-metastatic curatively resected GBC [24]. Only 12 of these patients had negative resection margin. Three patients had stage II disease, 15 had stage III and while the rest were stage IV. Median RT dose was 45 Gy/25 fractions (range 39.6-60 Gy). Concurrent bolus/continuous 5-FU were given to 18 of this cohort of patients. Median survival and DFS for the entire population were 1.9 years and 1.6 years respectively. OS and DFS at 2-year for the entire cohort was 55% and 50% respectively. Most of the patients had nausea and vomiting which were managed with antiemetic.
Gold DG et al., had retrospectively reviewed the effects of adjuvant concurrent chemoradiation in 25 patients of radical cholecystectomy for GBC and compared it with those 48 patients where no adjuvant treatment was offered after curative surgery [25]. Majority were stage I (59%) while the remaining had stage II disease. All the patients who had received CCRT had stage II disease. All the patients had R0 resection. Bolus 5-FU was given for first three days and last three days of RT. Median RT dose was 50.4 Gy in 30 fractions (range 19.75 to 54 Gy). OS at 2-years for the entire cohort of 73 patients was 75%. On subset analysis median OS for CCRT arm was 4.8 years while surgery alone arm was 4.2 years (p=0.56). On multivariate analysis, after adjusting for stage and histology, adjuvant CCRT was a significant predictor of improved OS (hazard ratio for death 0.3; 95% confidence interval, 0.13-0.69: p=0.004). There were two treatment related deaths.
Unlike the above trials, our study is based on stage II and stage III patients. All the studies had used 5-FU based agents as concurrent chemotherapy. To the best of our knowledge, this is the only study where CDDP has been used concurrently with RT for stage II and III GBC. Unlike 5-FU based chemotherapy, CDDP causes less acute side effects. Most of our patients had tolerated the treatment very well. OS and DFS for stage II and III patients of ours did not differ significantly probably because of low sample size. Compared to historical data our cohort of patients had comparable survival outcome. Due to small sample size we did not do a univariate analysis to analyse the prognostic factors which adversely affected the survival outcome. However, all the four patients who had failed had positive PNI and/or LVI or had advanced stage. Port site recurrence is a well accepted fact after laparoscopic surgery. Our patient failed at it probably because we did not include it in RT portals. In the hindsight, we wonder that the patients with poor prognostic factors would have probably done better had they received higher dose of RT i.e., 50.4-54 Gy instead of 45 Gy.
It is important to note that most of the previous trials were underpowered and included patients with all the stages of GBC. There is lots of heterogeneity in the treatment offered to these patients. Radiotherapy dose and chemotherapy schedules varied significantly. Therefore, it is difficult to draw any meaningful conclusions from the previous studies. All the patients in our study had the same treatment and were done for only stage II and III GBC.
Limitation
The most important caveat of our study was that it was a retrospective trial with a small sample size. Another pertinent problem with this study is that the follow-up period was small.
A multicentric study with an adequate sample size may be carried out prospectively to authenticate the results of this study.
Conclusion
The trial showed that CDDP based CCRT in adjuvant setting of curative cholecystectomy for GBC had survival outcomes which were comparable to 5-FU based CCRT with better compliance and less toxicity.