Case Report
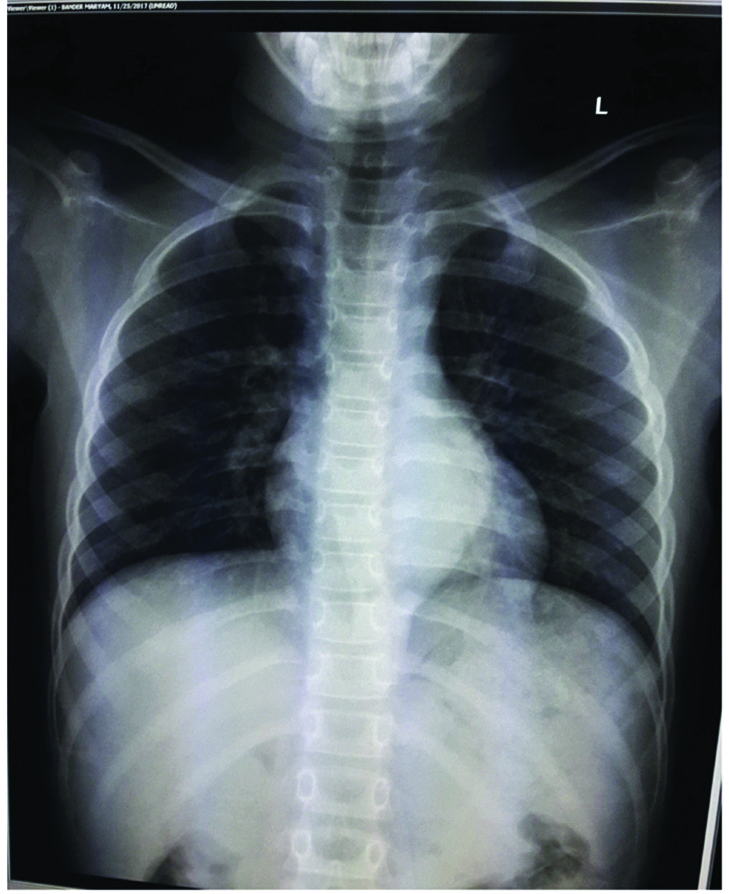
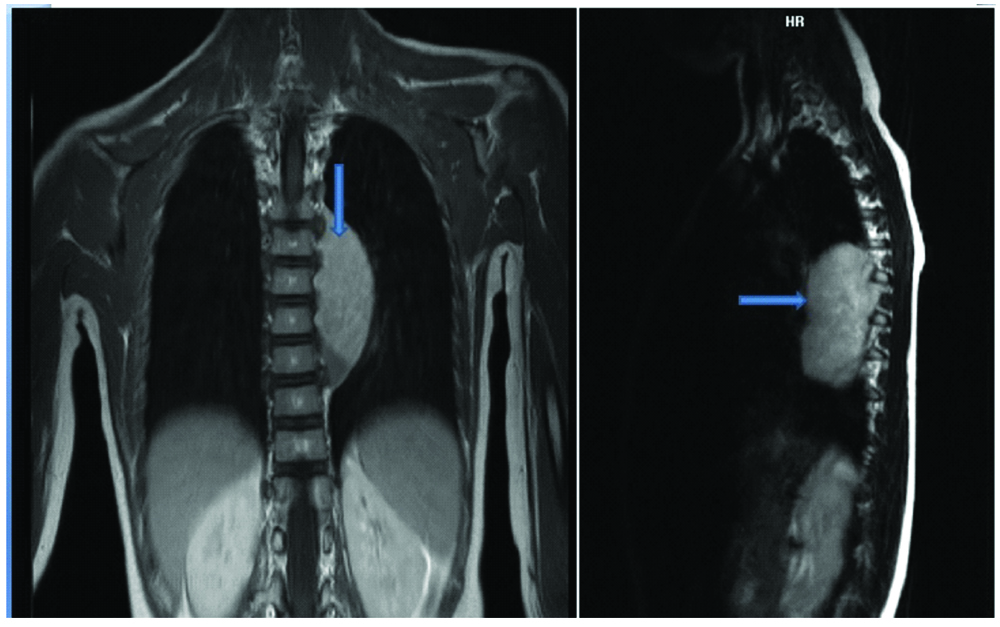
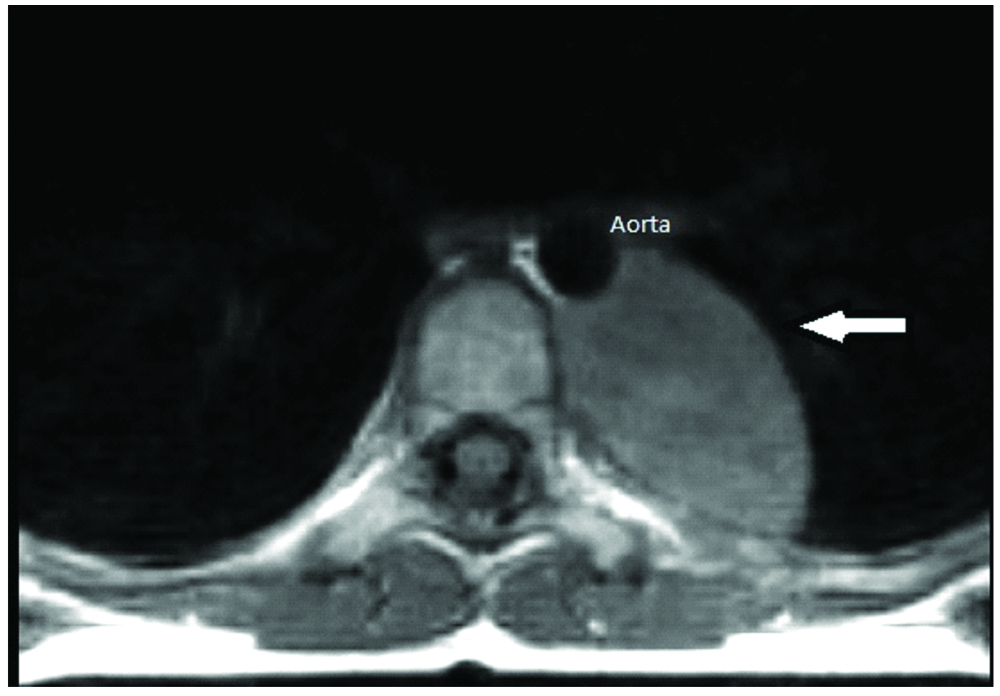
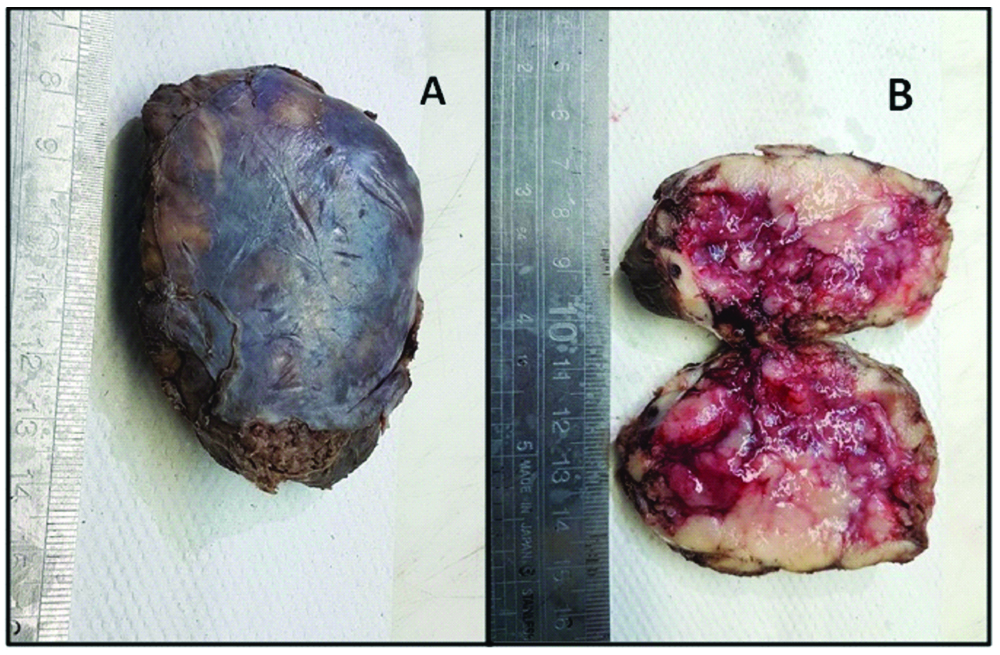
An eight-year-old girl presented with the chief complaints of running nose, cough and shortness of breath for three weeks to a private hospital. There was no significant past/family history. Her routine haematological and biochemical investigations were normal. Chest X-ray examination showed a left paraspinal well-defined density in the retrocardiac region. [Table/Fig-1]. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) of the chest was performed after examining the abnormal chest X-ray which revealed a well-defined mass in the left posterior mediastinal paraspinal region extending from T5 to T11 measuring 9×3.5×3 cm, contacting (contact <50%) the aorta [Table/Fig-2a,b] There was no intraspinal involvement or extension towards exit foramina. No intramedullary or intradural enhancing lesion was seen. Hence there were no Image defined Risk factors (IDRF) [1]. Considering the age and location of tumour, provisional diagnosis of a nerve sheath/sympathetic-parasympathetic ganglion tumour/germ cell tumour/lymphoma was made. Patient was referred to the paediatric surgery department of our hospital where surgery was performed and mass was removed by left thoracotomy. Gross examination showed an encapsulated nodular greyish brown mass measuring 7×5×2.5 cm [Table/Fig-3a]. Cut section was pale-white solid and nodular with foci of cystic and haemorrhagic areas [Table/Fig-3b]. There were no lymph nodes identified. Microscopic examination revealed a vaguely nodular tumour composed of small round blue cells with slight variation in size, hyperchromatic nuclei and little amount of cytoplasm, with occasional Homer Wright rosette formation (40% of tumour) surrounded by areas of mature well-differentiated ganglion cells with Schwannianstroma and neuropils (60% of tumour) [Table/Fig-4,5 and 6]. Mitosis Karyorrhexis Index (MKI) was low around 50/5000 cells with 0-1 mitosis/high power field. Foci of haemorrhage, necrosis and calcification were also seen. Mature lymphocytes were present in and around the tumour. Tumour cells reached up to the surgical margin. Immunohistochemistry exhibited Neuron Specific Enolase (NSE), synaptophysin positivity in small neuroblastic cells. Schwannianstroma was S100 positive and ganglion cells were S100 and synaptophysin positive [Table/Fig-7,8]. Tumour was negative for cytokeratin, EMA, CD45, Desmin, Vimentin, CD3, CD20, HCG. A final diagnosis of GNB, nodular (differentiating, Schwannianstroma rich) was made. Serum ferritin, Lactate Dehydrogenase (LDH), urine catecholamine metabolites (homovanillic acid, vanillylmandelic acid) were within normal range. Bone marrow examination of iliac crest and bone scan were normal. No other evidence of lymph node or organ metastasis was found. It was stage 1 as per International Neuroblastoma Risk Group (INRG) and International Neuroblastoma Staging System (INSS) [1,2]. Favourable prognostic factors for the patient were Stage 1 differentiating tumour, low MKI, normal ferritin, LDH. Unfavourable prognostic factor was age>5 years. Patient is doing well after complete removal of mass and is under follow-up with six monthly CT chest and blood biochemistry.
X-ray showing a left paraspinal well-defined density in the retrocardiac region.
Coronal and sagittal MR Images showing left paraspinal posterior mediastinal soft tissue mass (arrow) extending from T5 to T11 with no sizable intraspinal component or extension.
Axial T1 weighted MR Image showing left posterior mediastinal paraspinal mass (arrow) contacting (contact <50%) the aorta.
An encapsulated greyish brown mass measuring 7×5×2.5 cm. (1a) Cut section showing pale-white solid and nodular with foci of cystic and haemorrhagic areas.
Small round blue cells and occasional Homer Wright rosette (arrow) formation. (H&E, 100X).
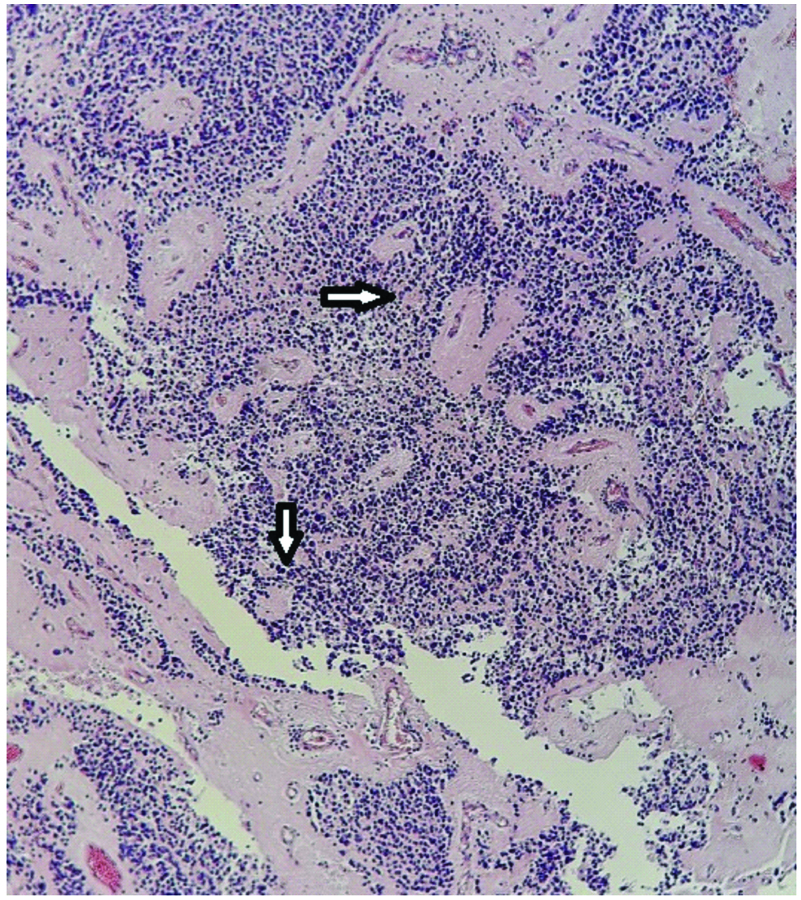
Small round blue cells with slight variation in size and Homer Wright rosette formation (arrow). (H&E, 200X)
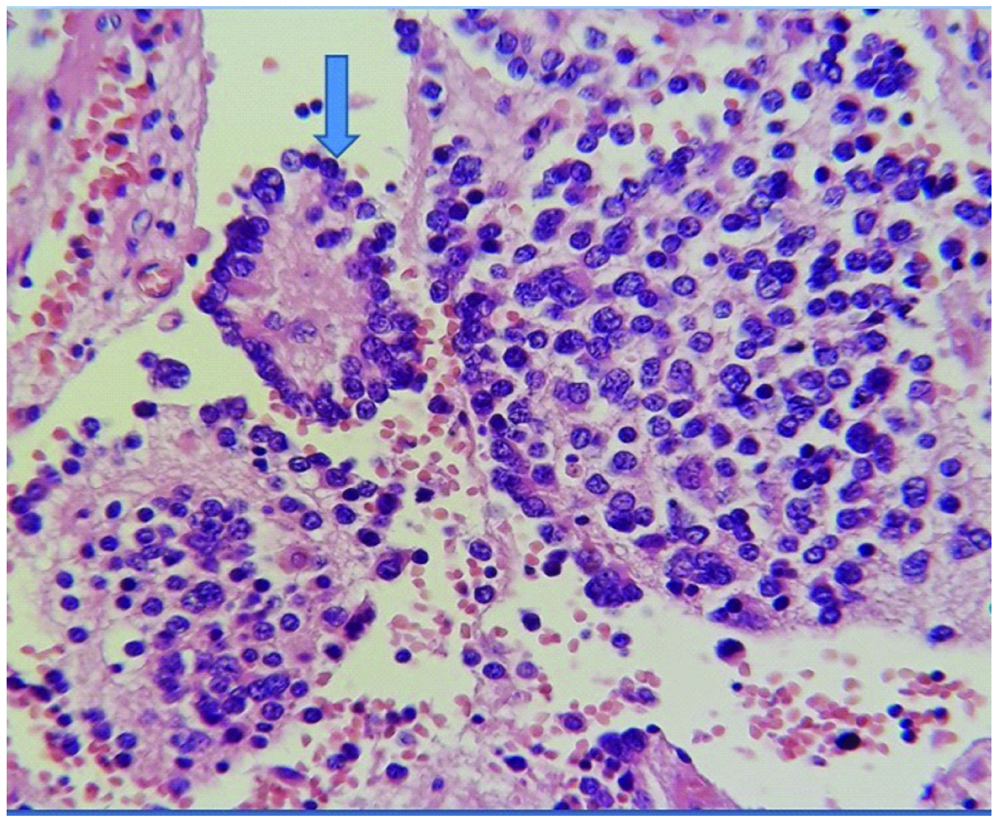
Surrounding tissue composed of Schwannianstroma and neurophils with areas of mature well-differentiated ganglion cells (arrow). (H&E, 200X).
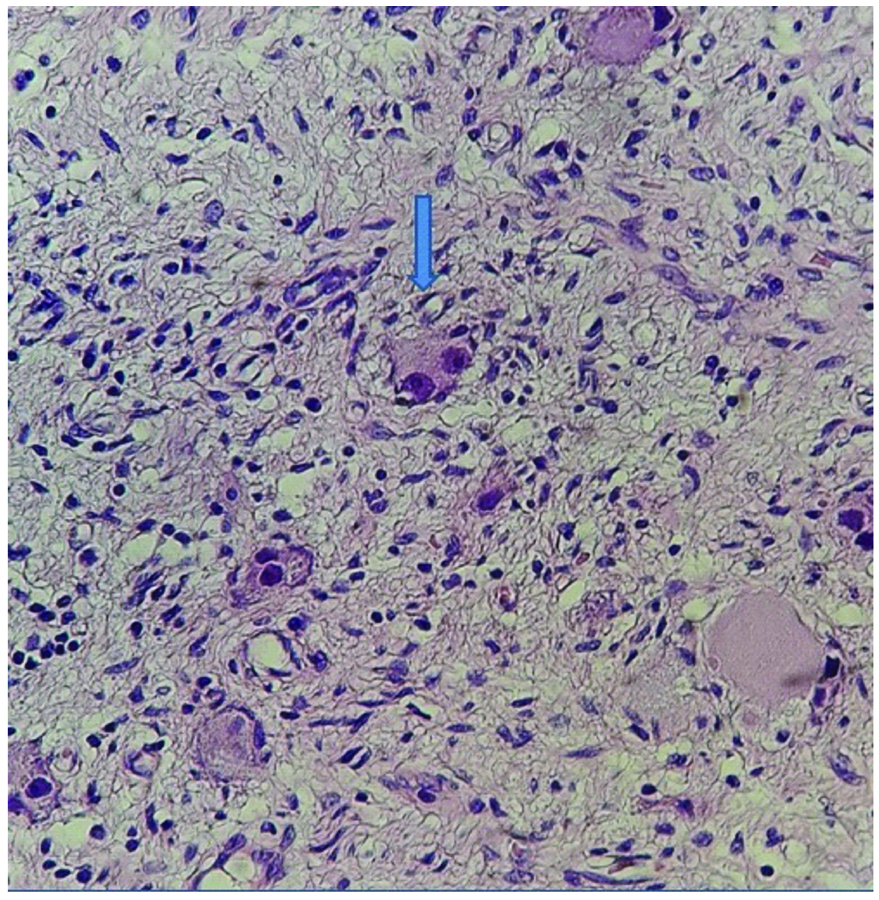
Schwannianstroma showing S100 positivity (40X).
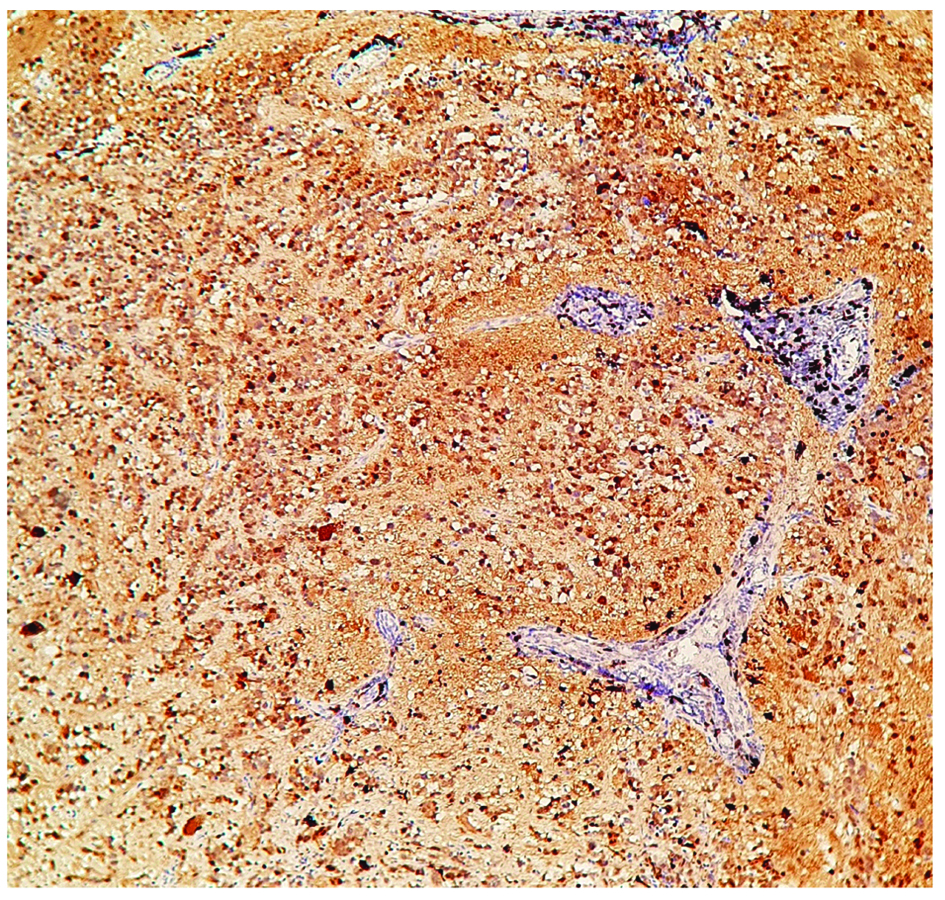
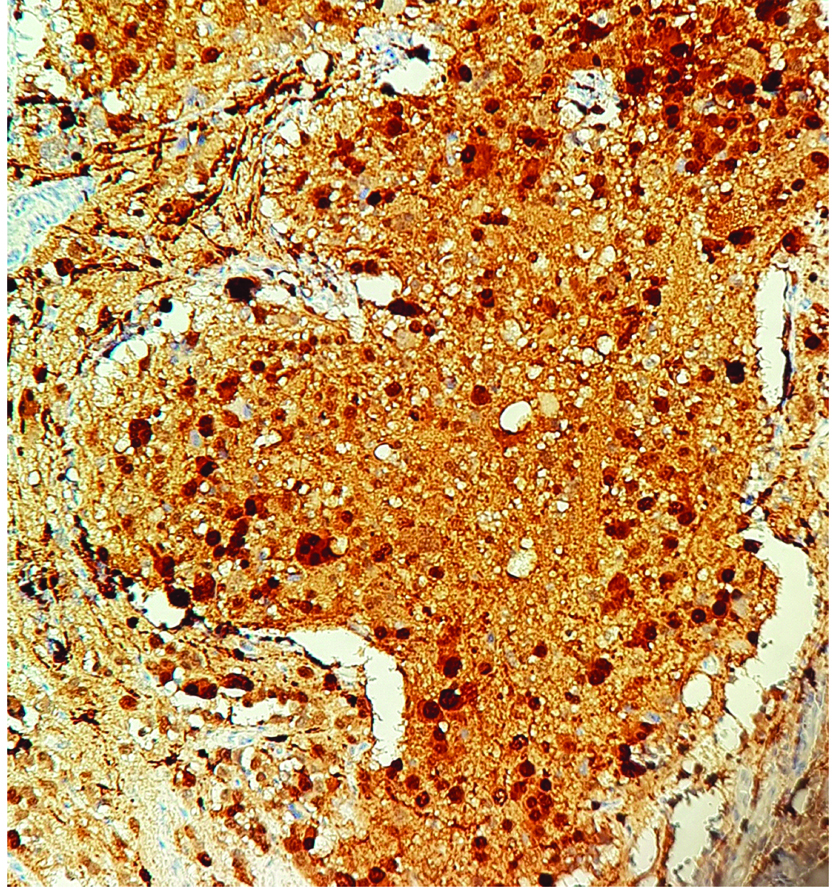
Synaptophysin positivity in small neuroblastic cells. (100X)
Discussion
The nodular variant of Ganglioneuroblastoma (GNB) is a rare histologic subtype in a spectrum of neuroblastic tumours, which are common in children of less than four years and is characterised by one or more macroscopic nodules of neuroblastoma within a ganglioneuromatous component. They exihibit biologically and clinically non-aggressive (Schwannianstroma-rich and stroma-dominant) components combined with aggressive nodular (Schwannianstroma-poor) components [3,4].
Ganglioneuroblastoma arises from sites of naturally occurring sympathetic tissue i.e., the adrenal medulla (35% of cases), extra adrenal retroperitoneum (30%-35%), and posterior mediastinum (20%). Less common sites are the neck (1%-5% of cases) and pelvis (2%-3%). These are tumours of children and 95% of cases are diagnosed by 10 years of age [5,6].
Histopathological examination is most important for the diagnosis and evaluation of these tumours with evolving classifications which endeavour to determine the prognosis of these tumours [7].
The International Neuroblastoma Pathology Classification proposed by the International Neuroblastoma Pathology Committee (INPC) [5] with the adoption of the original Shimada system [6] describes four categories of peripheral Neuroblastic (NB) tumours: Neuroblastoma (NB) (Schwannianstroma-poor); GNB, intermixed (Schwannianstroma-rich); Ganglioneuroma (GN) (Schwannianstroma-dominant); and GNB, nodular (Schwannianstroma-rich/stroma dominant and stroma-poor).
Ganglioneuroblastoma is considered to have intermediate malignancy potential, categorised as nodular (GNBn) and intermixed (GNBi). GNBn is a rare histologic subtype in this spectrum and represents 5-10% of NB tumours, characterised by one or more macroscopically visible nodules of NB showing neutrophils formation surrounded by well-differentiated Schwannian-type stroma with ganglion cells. Neuroblasts are positive for NSE, ALK 1, synaptophysin and chromogranin. Schwannianstroma is S100 and Galectin 3 positive. Ganglion cells are S100, synaptophysin, Glial Fibrillary Acidic Protein (GFAP) positive [8]. Although there are long studies on GNBn [4,9,10], there are only a few case reports of mediastinal GNBn in relatively older children [11,12].
Neuroblastoma tumours are divided into Favourable Histology (FH) or Unfavourable Histology (UH) groups depending on morphologic features (grade of neuroblastic differentiation-differentiating, poorly differentiated or undifferentiated and mitosis-karyorrhexis index [MKI], patient age). GNBi and GN were classified into an FH group, whereas GNBn tumours were classified into an UH group considered to be associated with aggressive clinical behaviour [6].
Later on, GNBn subtype got divided into two prognostic subsets-Favourable Nodule (FN) and Unfavourable Nodule (UnFN) [8,13]. FN subset is composed of Schwannian-rich, stroma-dominant component, with nodules composed of: {a} poorly differentiated or differentiating neuroblastoma: MKI (count of cells undergoing mitosis or karryhorexis, based on 5,000 cell count from random fields) ≤200 and age <1.5 years; or {b} differentiating neuroblastoma having MKI <100, age 1.5-5 years. This is based on eight year event free survival of 86.1% and overall survival of 90.5% [13].
Unfavourable subset is composed of UnFN which is: {a} any NB with MKI >200, any age; or {b} any NB with MKI 100-200, age >1.5 years; {c} undifferentiated NB, any age; or {d} poorly differentiated neuroblastoma, age >1.5 years; or {e} any neuroblastoma, age >5 years. This is based on eight year event free survival of 32.2% and overall survival of 33.2% [9,13,14].
This patient was little older for GNBn as majority of cases are between 1.8 to 3.4 years of age and hence, considered as UnFN subset [9,14]. However histologically, nodules showed differentiating NB having MKI <100. Also, clinically it was found to be stage 1.
INSS gives staging based on clinical, radiologic, and post surgical features, divided in three stages. Tumours that are grossly completely resected and have no positive lymph nodes or metastases are stage 1. Extent across the midline with ipsilateral and contralateral regional lymph node involvement decides stage 2 and 3. Presence of metastasis is stage 4. But INSS is not suitable for pre-treatment staging and risk assessment [1]. Therefore, INRG system was developed for evaluation of pre-treatment risk factors which requires diagnosis using histopathology, urine or serum catecholamines, imaging studies with CT/MRI for Image Defined Risk Factors (IDRF), Iodine-123 metaiodobenzylguanidine (MIBG) scintigraphy. INRG has four stages L1, L2, M and MS. L1 and L2 is localised tumour with and without IDRF respectively. M is metastasis, MS is metastasis in children less than 18 months with metastasis to skin, liver, bone marrow [2].
The Neuroblastoma (NB) component is thought to have a clonal origin and determines the clinical behaviour of the tumour [7,8]. However a recent study shows that the NB and GN components of GNBn are genetically similar, the ganglion cells and neuroblastoma cells share genetic changes. However, more studies are needed to prove this [10].
Prognosis of NB is determined by age, ferritin, LDH, stage (1 to 4) and pathological features (histological category-FH, UH and grade of tumour differentiation- differentiating, poorly differentiated or undifferentiated), MYC-N amplification, ploidy-hyperdiploid, diploid or hypodiploid and 11q, 1p and 17q abnormalities and these factors are also relevant for GNBn. MYC-N amplification correlates with unfavourable pathology, older age, advanced stage and poor prognosis but has been found to be less common (<2%) in GNBn compared to NB. Also, high MKI is prognostically relevant only in young patients and LDH is not prognostic in GNBn [9]. Diploid DNA content is more common in older patients, and it is associated with MYC-N amplification and worse outcome. Hyperdiploid, non MYC-N amplified tumours have been shown to have a favourable outcome in children 12-18 months of age [2,9,15].
Neuroblastic tumours have varied clinical behaviour. They are often present with pain and are caused by either local effects from the primary tumour or metastatic disease. Other presenting symptoms include malaise, irritability, weight loss, shortness of breath (from a large mediastinal or abdominal tumour), and peripheral neurologic deficit (from neural foraminal invasion and nerve compression by tumour). Vasoactive Intestinal Peptide (VIP) may be secreted by the tumour and may result in watery diarrhea, hypokalemia, and acidosis [16].
Radiographs may show a posterior mediastinal mass, calcification seen in 30% cases. CT is the most commonly used imaging modality for assessment of neuroblastic tumours [15]. The most common site for metastasis in GNB is bone (Hutchinson’s syndrome) and liver (Massive liver metastases in infants is called Pepper syndrome). Skin metastases are common in children <1 year, which is darkly, pigmented masses resembling blueberries (‘blueberry muffin’ syndrome) [17].
Low risk tumours are usually treated by complete resection alone. Intermediate risk patients are treated by resection and chemotherapy. High risk and stage 4 patients are treated by chemotherapy along with radiation [18].
Conclusion
GNBn is a rare subtype of NB tumours with an evolving staging and histological classification. Its classified into an unfavourable histology group but has been divided into two prognostic subsets-FN and UnFN. Prognosis is determined by age, ferritin, stage and pathological features (grade of tumour differentiation), MYC-N amplification, ploidy and 11q, 1p and 17q abnormalities. Treatment varies according to risk category.
[1]. Monclair T, Brodeur GM, Ambros PF, Brisse HJ, Cecchetto G, Holmes K, The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force reportJ Clin Oncol 2009 27(2):298-303.10.1200/JCO.2008.16.687619047290 [Google Scholar] [CrossRef] [PubMed]
[2]. Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatmentJ Clin Oncol 1993 11:1466-77.10.1200/JCO.1993.11.8.14668336186 [Google Scholar] [CrossRef] [PubMed]
[3]. Adams A, Hochholzer L, Ganglioneuroblastoma of the posterior mediastinum: a clinicopathologic review of 80 casesCancer 1981 47:373-81.10.1002/1097-0142(19810115)47:2<373::AID-CNCR2820470227>3.0.CO;2-O [Google Scholar] [CrossRef]
[4]. Shimada H, Ambros IM, Dehner LP, Hata J, Joshi VV, Roald B, The International Neuroblastoma Pathology Classification (the Shimada system)Cancer 1999 86(2):364-72.10.1002/(SICI)1097-0142(19990715)86:2<364::AID-CNCR21>3.0.CO;2-7 [Google Scholar] [CrossRef]
[5]. Joshi VV, Peripheral neuroblastic tumours: pathologic classification based on recommendations of International Neuroblastoma Pathology Committee (modification of Shimada classification)Pediatr Dev Pathol 2000 3:184-89.10.1007/s10024005002410679039 [Google Scholar] [CrossRef] [PubMed]
[6]. Shimada H, Umehara S, Monobe Y, Hachitanda Y, Nakagawa A, Goto S, International Neuroblastoma Pathology Classification for prognostic evaluation of patients with peripheral neuroblastic tumours: a report from the Children’s Cancer GroupCancer 2001 92:2451-61.10.1002/1097-0142(20011101)92:9<2451::AID-CNCR1595>3.0.CO;2-S [Google Scholar] [CrossRef]
[7]. Joshi VV, Cantor AB, Altshuler G, Larkin EW, Neill JS, Shuster JJ, Recommendations for modification of terminology of neuroblastic tumours and prognostic significance of Shimada classificationCancer 1992 69:2183-96.10.1002/1097-0142(19920415)69:8<2183::AID-CNCR2820690828>3.0.CO;2-C [Google Scholar] [CrossRef]
[8]. Umehara S, Nakagawa A, Matthay KK, Lukens JN, Seeger RC, Stram DO, Histopathology defines prognostic subsets of ganglioneuroblastoma, nodularCancer 2000 89:1150-61.10.1002/1097-0142(20000901)89:5<1150::AID-CNCR25>3.3.CO;2-Z [Google Scholar] [CrossRef]
[9]. Angelini P, London WB, Cohn SL, Pearson AD, Matthay KK, Monclair T, Characteristics and outcome of patients with ganglioneuroblastoma, nodular subtype: a report from the INRG projectEur J Cancer 2012 48:1185-91.10.1016/j.ejca.2011.10.03722137163 [Google Scholar] [CrossRef] [PubMed]
[10]. Angelini P, Baruchel S, Marrano P, Irwin MS, Thorner PS, The neuroblastoma and ganglion components of nodular ganglioneuroblastoma are genetically similar: evidence against separate clonal originsModern Pathology 2015 28:166-76.10.1038/modpathol.2014.9025081755 [Google Scholar] [CrossRef] [PubMed]
[11]. Fatimi SH, Bawany SA, Ashwaq A, Ganglioneuroblastoma of the posterior mediastinum: a case reportJ Med Case Reports 2011 5:32210.1186/1752-1947-5-32221781292 [Google Scholar] [CrossRef] [PubMed]
[12]. Guarino S, Astini C, Howard JP, Colombelli V, Large mediastinal nodular ganglioneuroblastoma in a child from AfricaAnn Ital Chir 2012 83:543-46. [Google Scholar]
[13]. Peuchmaur M, d’Amore ES, Joshi VV, Hata J, Roald B, Dehner LP, Revision of the International Neuroblastoma Pathology Classification: confirmation of favourable and unfavourable prognostic subsets in ganglioneuroblastoma, nodularCancer 2003 98(10):2274-81.10.1002/cncr.1177314601099 [Google Scholar] [CrossRef] [PubMed]
[14]. Okamatsu C, London WB, Naranjo A, Hogarty MD, Gastier-Foster JM, Look AT, Clinicopathological characteristics of ganglioneuroma and ganglioneuroblastoma: a report from the CCG and COGPediatr Blood Cancer 2009 53(4):563-69.10.1002/pbc.2210619530234 [Google Scholar] [CrossRef] [PubMed]
[15]. George RE, London WB, Cohn SL, Maris JM, Kretschmar C, Diller L, Hyperdiploidy plus nonamplified MYCN confers a favourable prognosis in children 12 to 18-month-old with disseminated neuroblastoma: A Pediatric Oncology Group studyJ Clin Oncol 2005 23(27):6466-73.10.1200/JCO.2005.05.58216116152 [Google Scholar] [CrossRef] [PubMed]
[16]. Geoerger B, Hero B, Harms D, Grebe J, Scheidhauer K, Berthold F, Metabolic activity and clinical features of primary ganglioneuromasCancer 2001 91:1905-13.10.1002/1097-0142(20010515)91:10<1905::AID-CNCR1213=3.0.CO;2-4 [Google Scholar] [CrossRef]
[17]. Lucky AW, McGuire J, Komp DM, Infantile neuroblastoma presenting with cutaneous blanching nodulesJ Am Acad Dermatol 1982 6:389-91.10.1016/S0190-9622(82)70034-9 [Google Scholar] [CrossRef]
[18]. Okamatsu C, London WB, Naranjo A, Hogarty MD, GastierFoster JM, Look AT, Clinicopathological characteristics of ganglioneuroma and ganglioneuroblastoma: a report from the CCG and COGPediatr Blood Cancer 2009 53(4):563-69. [Google Scholar]