Primary Epidural Intraspinal Haemangiopericytoma
Ameen Abdul Mohammad1, Bala Balaji Vosuri2, KVN Srikanth3
1 Consultant Neurosurgeon, Department of Neurosurgery, Aayush NRI LEPL Healthcare Ltd., Vijayawada, Andhra Pradesh, India.
2 Junior Consultant, Department of Neurosurgery, Aayush NRI LEPL Healthcare Ltd., Vijayawada, Andhra Pradesh, India.
3 Consultant Pathologist, Department of Pathology, Aayush NRI LEPL Healthcare Ltd.,Vijayawada, Andhra Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Ameen Abdul Mohammad, Aayush NRI LEPL Healthcare Ltd., Vijayawada, Andhra Pradesh, India.
E-mail: drmaameen@gmail.com
Here, authors present a case report of a 25-year-old male who reported with low back pain with left lower limb radiculopathy for two years. Neurological examination revealed no focal neurological deficits, except for a positive Straight Leg Raise Test (SLRT) at 30°. Plain radiographs of the lumbar spine showed mild scalloping of the L3 vertebral body and Magnetic Resonance Imaging (MRI) lumbar spine showed an extradural space occupying lesion at the level of L3 vertebral body compressing the thecal sac and the left L3 nerve root. He underwent L2-4 median laminectomy without fusion and en bloc resection of the tumour. The histopathology was reported as a Haemangiopericytoma (HPC). Patient was referred for postoperative radiation therapy in view of high incidence of recurrence. Post radiation, at one year follow-up, MRI scan showed no signs of recurrence. Complete surgical removal of the tumour, followed by postoperative radiation therapy, appears to be the treatment of choice for such primary intraspinal haemangiopericytomas.
Epidural, Intraspinal, Primary haemangiopericytoma
Case Report
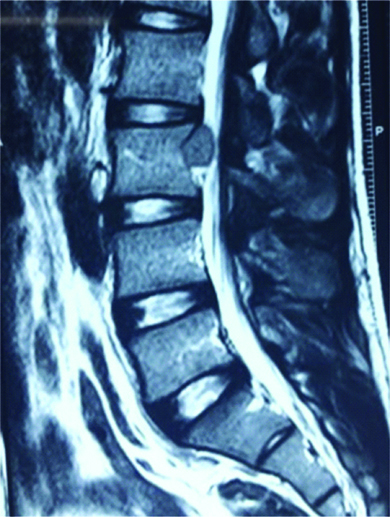
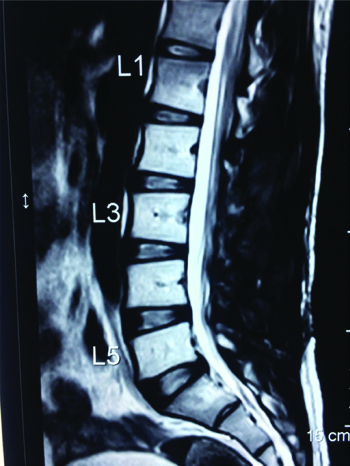
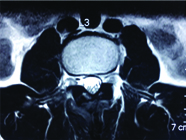
A 25-year-old male patient, a car mechanic by occupation, presented with low back pain for two years with recent onset of left lower limb radiculopathy for three months. On examination, his left SLRT was positive at 30° but otherwise, local examination of the back revealed no abnormality and no focal neurological deficits were observed. Plain radiographs of the lumbar spine showed mild scalloping of the L3 vertebral body. MRI of the lumbar spine revealed an extradural space occupying lesion at the level of L3 vertebral body, which was hypointense on both T1 and T2 weighted sequences, causing compression over the thecal sac and the L3 nerve root on the left side [Table/Fig-1,2].
Preoperative Sagittal T2 weighted MRI showing an extradural space occupying lesion at the level of L3 compressing the thecal sac.
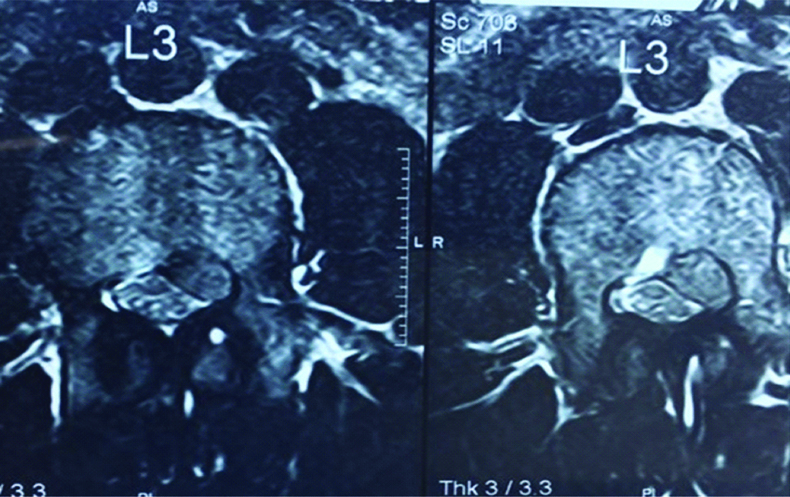
Preoperative axial T2 weighted MRI showing an extradural lesion on the left side compressing the thecal sac and causing severe left neural foraminal stenosis.
L2-4 median laminectomy without fusion and en bloc excision of the tumour through a midline lumbar incision was planned. Intraoperatively, the tumour was greyish white, measuring 3 cm×1.5 cm, well defined, firm, fleshy with moderate vascularity and was loosely adherent to the dural surface. The tumour was easily separated from the adjacent dura and excised completely. Postoperatively the left lower limb radiculopathy disappeared and there were no fresh deficits.
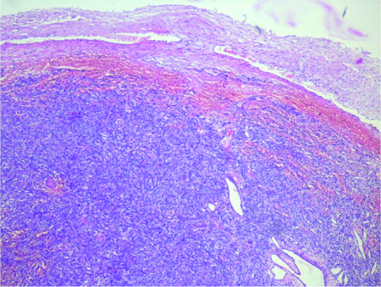
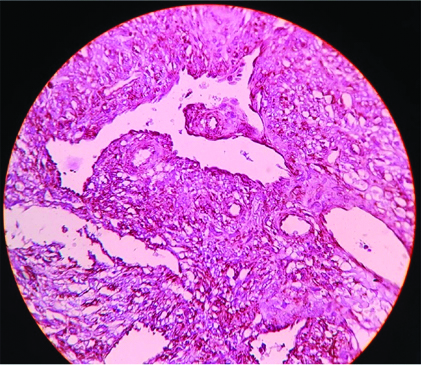
Histopathology was reported as a HPC [Table/Fig-3]. Immunohistochemistry [Table/Fig-4] proved positivity for Haematopoietic progenitor cell antigen, CD 34 and negativity for Epithelial Membrane Antigen (EMA). The Ki 67 labelling index was 1% indicating a low proliferation rate of the tumour cells. Summarising all these findings a final histological diagnosis of an HPC was made. The patient was referred for postoperative radiation therapy in view of high rate of recurrence of the tumour. After completion of the radiation therapy, he is presently on regular follow-up and one year postoperative MRI scan showed no evidence of recurrence [Table/Fig-5,6].
Light Microscopy with Haematoxylin and Eosin Stain (10x magnification) showing elongated cells with spindle shaped nuclei arranged in a storiform pattern with intratumoralstaghorn vessels in a peritheliomatous pattern.
IHC of Haemangiopericytoma. Haematoxylin and Eosin Staining (40x Magnification) Vimentin showing strong positivity in the tumor cells, vascular endothelial cells and vascular smooth muscle cells. Type III Intermediate Filament (IF) protein expression is seen over the tumor cells of mesenchymal cell origin.
One-year postoperative follow-up T2 weighted sagittal MRI showing postoperative changes and no signs of recurrence.
One-year postoperative follow-up T2 weighted axial MRI showing postoperative changes and no signs of recurrence.
Discussion
HPCs are rare tumours accounting for <1% of all central nervous system tumours and their incidence in the intraspinal compartment is rarer [1]. They are soft tissue tumours originating from pericytes in the walls of the blood vessels [2]. They include a variety of lesions comprising certain morphological characteristics; a monotonous appearance at low-power examination, moderate to high cellularity, and numerous, variably thick-walled, branching ‘staghorn’ vessels [3]. In 2016, World Health Organisation classified these to be “mesenchymal nonmeningothelial” tumours and subdivided them as fibroblastic where there is no evidence of pericytic differentiation. Those with pericytic differentiation have been classified as Grade II tumours. Anaplastic Grade III tumours are those with infiltrative margins, high cellularity, nuclear pleomorphism, areas of tumour necrosis, and increased mitotic index (>4 mitoses/10×high-powered field) [4]. The present was a Grade II tumour based on the above classification. Patients usually suffer localised back pain with or without radiculomyelopathy.
MRI remains the investigation of choice for identifying spinal HPCs. Both intracranial and intraspinal HPCs share the same MRI findings. They appear isointense on T1-weighted imaging and hyperintense on T2-weighted imaging and they enhance homogeneously on contrast administration. Long-standing tumours may even erode the adjacent bony structures like the lamina, pedicle, neural foramen and even paraspinal muscle. The occasional dumb bell appearance of the tumour is seen when there is an extension into the neural foramen [5], usually in intradural lesions.
En bloc resection of the tumour is recommended by most neurosurgeons to prevent local recurrence and to minimise blood loss [6]. However, an intratumoural decompression and piecemeal excision by staying within the arachnoid plane becomes necessary when there is severe neural compression. However, a complete resection is always necessary to prevent further recurrences.
There are a number of case series of meningeal HPCs in the literature. However, there have not been many case reports of intraspinal involvement of such tumours. In 1961, Kruse F Jr, was the first to report an intradural spinal HPC [7]. Since then, there have been many case series of such intraspinal involvement of HPCs, most of which have been reported to be extradural in origin. However, primary involvement of the intraspinal compartment has been reported in only a few among all these cases [5].
HPCs can involve the spine either intradurally or extradurally, and the outcome in both the scenarios has been found similar. However, the disease-free survival was longer in patients with intradural lesions that those with extradural ones [8]. Chou CW et al., reported an overall recurrence rate of 53%, the incidence of which was higher in those in the extradural compartment than its intradural counterpart [9].
Though surgical excision of the lesion offers good local control for many years, an addition of postoperative radiotherapy helps in a better outcome on a long term basis. The complications associated with adjacent tissue damage, particularly the spinal cord can be minimised by intensity modulated radiotherapy and fractionated stereotactic radiotherapy with high radiation doses. In cases with prior radiotherapy, radiosurgery and intensity modulated radiotherapy can be considered, where higher and more conformal doses of radiation can give better local control. However, the role of chemotherapy has shown disappointing results [10].
Conclusion
Intraspinal Haemangiopericytomas are rare tumours and complete excision followed by radiotherapy gives a long-term disease-free period. The overall progression-free survival rates have been promising lately, but the treatment of Spinal HPCs still remains a great challenge.
[1]. Ramdasi RV, Nadkarni TD, Goel NA, Hemangiopericytoma of the cervical spineJ Craniovertebr Junction Spine 2014 5(2):95-98.10.4103/0974-8237.13920925210342 [Google Scholar] [CrossRef] [PubMed]
[2]. Stout AP, Murray MR, Hemangiopericytoma: a vascular tumor featuring Zimmermann’s pericytesAnn Surg 1942 116(1):26-33.10.1097/00000658-194207000-0000417858068 [Google Scholar] [CrossRef] [PubMed]
[3]. Gengler C, Guillou L, Solitary fibrous tumour and haemangiopericytoma: evolution of a conceptHistopathology 2006 48(1):63-74.10.1111/j.1365-2559.2005.02290.x16359538 [Google Scholar] [CrossRef] [PubMed]
[4]. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summaryActa Neuropathol 2016 131(6):803-20.10.1007/s00401-016-1545-127157931 [Google Scholar] [CrossRef] [PubMed]
[5]. Zhao Y, Zhao JZ, Clinical and pathological characteristics of primary intraspinal hemangiopericytoma and choice of treatmentChin Med J (Engl) 2007 120(2):115-19.10.1097/00029330-200701020-00008 [Google Scholar] [CrossRef]
[6]. Kim JH, Jung H-W, Kim Y-S, Kim CJ, Hwang S-K, Paek SH, Meningeal hemangiopericytomas: long-term outcome and biological behaviorSurg Neurol 2003 59(1):47-54.10.1016/S0090-3019(02)00917-5 [Google Scholar] [CrossRef]
[7]. Kruse F Jr, Hemangiopericytoma of the meninges (angioblastic meningioma of Cushing and Eisenhardt). Clinico-pathologic aspects and follow-up studies in 8 casesNeurology 1961 11:771-77.10.1212/WNL.11.9.77113754624 [Google Scholar] [CrossRef] [PubMed]
[8]. Betchen S, Schwartz A, Black C, Post K, Intradural hemangiopericytoma of the lumbar spine: case reportNeurosurgery 2002 50(3):654-57.10.1227/00006123-200203000-0004511841738 [Google Scholar] [CrossRef] [PubMed]
[9]. Chou CW, Hsu SP, Lin SC, Chen MH, Shih YH, Lee LS, Primary intradural hemangiopericytoma with intramedullary invasionJ Chin Med Assoc 2009 72(10):536-41.10.1016/S1726-4901(09)70424-1 [Google Scholar] [CrossRef]
[10]. Galanis E, Buckner JC, Scheithauer BW, Kimmel DW, Schomberg PJ, Piepgras DG, Management of recurrent meningeal hemangiopericytomaCancer 1998 82(10):1915-20.10.1002/(SICI)1097-0142(19980515)82:10<1915::AID-CNCR15>3.0.CO;2-W [Google Scholar] [CrossRef]