Implantable Cardioverter and Defibrillators (ICD) prolong lifespan in patients at high risk of tachyarrhythmia, by their ability to appropriately detect and terminate it on the basis of Tachycardia Detection Rate (TDR). However, ICD patients often have Slow ventricular tachycardia (VT) defined as those below the detection limit of ICD. Such Slow VT’s are not entirely benign, leading to frequent emergency visits, in-hospital admissions, rate-induced cardiomyopathy and rarely death. Lowering the TDR to detect such arrhythmias has the potential of increasing inappropriate shocks which can be catastrophic. Although such slow VT’s are usually terminated by anti-tachycardia pacing, it is not always so. Here, the authors describe two such cases of resistant Slow VT’s in post ICD patients which ultimately necessitated ICD shocks.
Case Report
Case-1
A 74-year-old male presented to the emergency department with complaints of palpitation for past two days. He had no associated complaints of chest pain, dyspnea, presyncope or syncope. He was a known case of ischaemic cardiomyopathy and a dual chamber Implantable Cardioverter Defibrillator (ICD) was implanted ten years back for secondary prevention of sudden cardiac death. He was a non smoker, non-alcoholic, non-diabetic with long-standing hypertension. Patient was asymptomatic during regular outpatient cardiology follow-up. His concurrent oral medications included carvedilol 12.5 mg twice daily, amiodarone 100 mg once daily, ramipril 5 mg and torsemide 20 mg both once daily and was compliant to these guidelines based therapies.
On examination, there was no evidence of haemodynamic instability with a blood pressure of 110/64 mmHg, heart rate of 140 beats/minute and a respiratory rate 16/minute. His cardiovascular system examination was within normal limits. Two-dimensional transthoracic echocardiography was done which suggested global left ventricular hypokinesia with a Left Ventricular Ejection Fraction (LVEF) of 34% (calculated by average of M-Mode and bi plane Simpson’s method) and mild mitral regurgitation.
His 12 lead electrocardiogram revealed a regular, wide QRS tachycardia with a left bundle branch block (LBBB) morphology and Atrioventricular (AV) dissociation suggestive of ventricular tachycardia at a rate of 110/minute [Table/Fig-1]. Routine blood parameters revealed a low serum potassium-3.1 mmol/L (normal-3.5-5.0 mmol/L), serum magnesium-2.2 mg/dL (range-1.6-2.3 mg/dL) and serum creatinine-1.5. Serial cardiac biomarkers (high sensitive Troponin-T repeated 6 hours apart) were negative, ruling out any evidence of active coronary ischaemia.
Twelve lead ECG showing Wide QRS tachycardia with left axis deviation, LBBB morphology and AV dissociation (arrows) suggestive of Ventricular tachycardia.
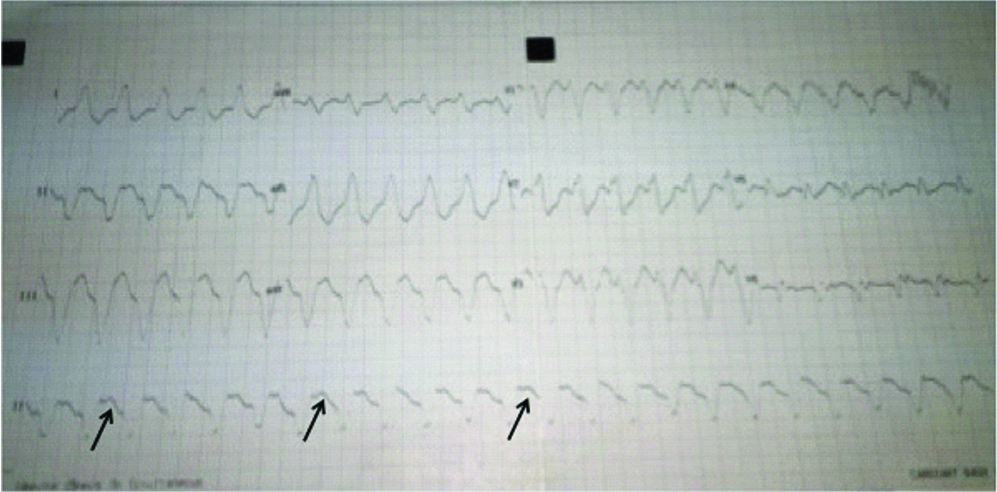
Intravenous Amiodarone was administered as 150 mg bolus followed by body weight based infusion. Simultaneous intravenous potassium correction was also initiated. However, even after six hours of Amiodarone infusion when VT persisted, intravenous lignocaine bolus and magnesium sulphate supplementation were added to the treatment regimen. Meanwhile, serum potassium levels got corrected to 4.2 mmol/L but despite all these measures VT continued. Surprisingly, patient remained in haemodynamically stable condition.
ICD interrogation suggested that it did not detect VT because it was programmed to detect VT at 171 beats per minute. ICD reprogramming was done to detect VT at 130 beats per minute. To terminate the VT, six cycles of Anti-Tachycardia Pacing (ATP) were delivered but failed to achieve restoration of sinus rhythm. Finally, an electrical shock of 36J was delivered from the ICD leading to reversion of normal sinus rhythm [Table/Fig-2].
Twelve lead ECG after ICD shock demonstrating sinus rhythm.
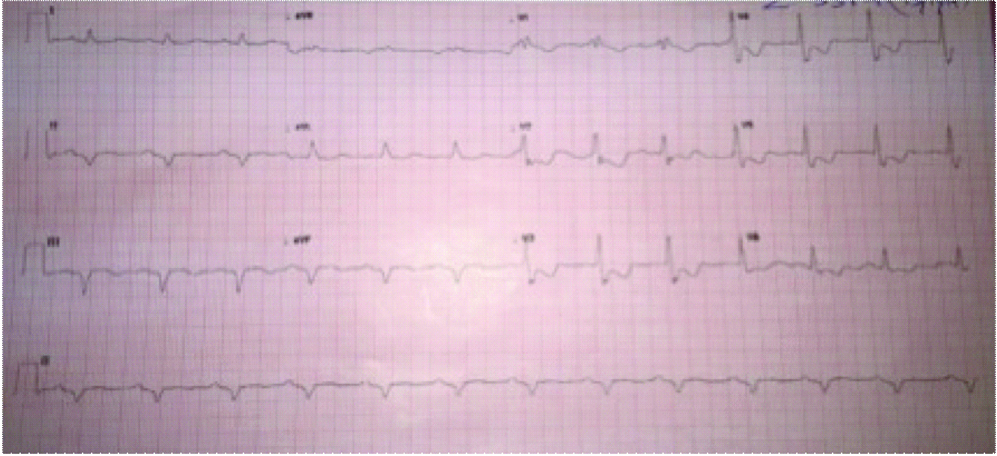
Patient was discharged the next day on same doses of carvedilol (12.5 mg twice daily) and amiodarone doses (100 mg once daily) and reduced torsemide dose (10 mg once daily). At second and four weeks follow-up, he continued to be in sinus rhythm without any ICD therapy and with normokalemia.
Case-2
A 37-year-old male presented in cardiology emergency department with complaints of palpitation, dyspnea and profuse sweating since one day. Patient had no additional complaints of any chest discomfort, presyncope or syncope. The dyspnea was of New York Heart Association (NYHA) Class III severity. He was an old case of ischaemic cardiomyopathy with a left ventricular ejection fraction 34% and mild eccentric mitral regurgitation with a single chamber ICD (Biotronic Inc, Lake Oswego, OR, USA) implanted two years back for refractory ventricular tachycardia. He was a non-hypertensive, non-diabetic, chronic smoker and tobacco chewer. His outpatient department records revealed that he was on treatment with metoprolol 100 mg once daily, amiodarone 100 mg twice daily, ramipril 5 mg once daily, torsemide 20 mg and spironolactone 50 mg.
On examination, he was haemodynamically stable with a blood pressure of 120/70 mm Hg, heart rate-128 beats/minute and respiratory rate of 20/minute. Chest auscultation revealed bilateral basal crepitations. His 12 lead electrocardiograms showed ventricular tachycardia at a rate of 128 beats/minute [Table/Fig-3]. Bedside two-dimensional echocardiography was suggestive of global Left Ventricular (LV) hypokinesia, LVEF of 30%, moderate eccentric mitral regurgitation. Blood investigation revealed serum potassium-3.8 mmol/L (range 3.5-5.0), serum magnesium-2.12 mg/dL (range 1.3-2.5) and serum creatinine-1.2 mg/dL. Negative serial cardiac biomarkers done six hours apart ruled out any cardiac ischaemia.
A 12 lead ECG suggestive of ventricular tachycardia with an Right Bundle Branch Block (RBBB) morphology with right axis deviation and AV dissociation (arrows).
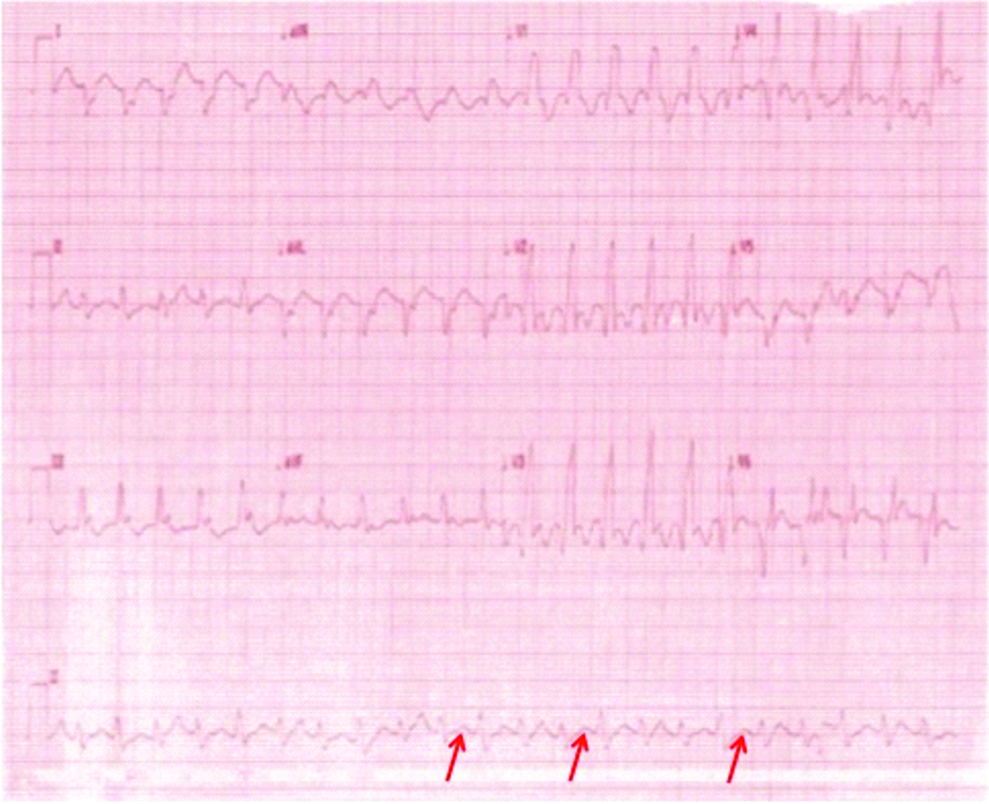
As a standard protocol, he was treated with intravenous amiodarone 150 mg bolus followed by intravenous weight based infusion. However, after six hours of amiodarone VT did not revert as in the previous case. Hence, intravenous lignocaine bolus followed by infusion and serum potassium correction was initiated but the VT was resistant to both of the measures.
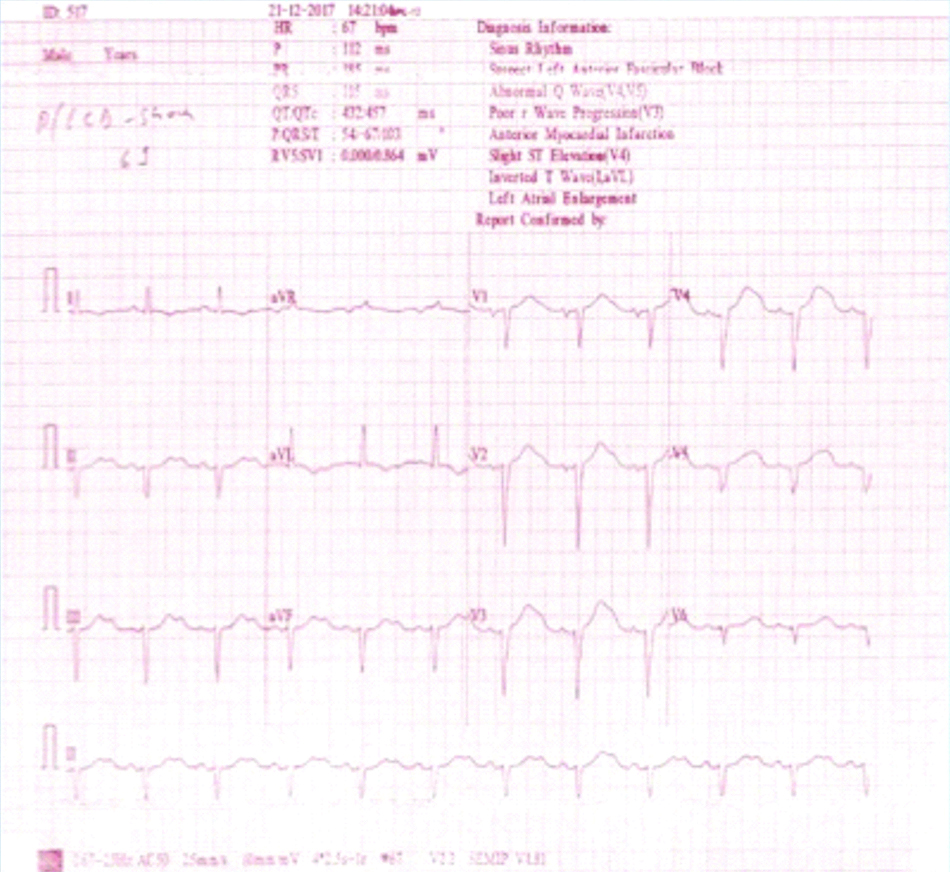
ICD did not detect VT because it was programmed to detect VT at 171 beats per minute. ICD reprogramming was done to detect VT at 120 beats per minute. Six cycles of ATP were delivered in burst followed ramp pattern. This was followed by an ICD shock of 16J which terminated the VT and sinus rhythm was restored [Table/Fig-4]. Patient was under observation for next 24 hours and discharged the next day with lower amiodarone dose (100 mg once daily). He remained free of VT and symptoms at four and eight weeks.
Sinus rhythm attained after ICD delivered a single shock.
Discussion
ICDs reduce mortality by terminating life-threatening ventricular tachyarrhythmia in patients who have been resuscitated from sudden cardiac death or are at high risk of developing them [1]. The primary mechanism of tachycardia detection by ICD’s is Tachycardia detection rate (TDR, many times expressed as cycle length) the cut-off point beyond which therapy (ATP or shock) is delivered by device. In clinical practice, TDR is usually programmed with a safety margin of 20 beats per minute below the clinical arrhythmia. Current generation ICD’s also employ additional parameters like morphology discrimination, onset characteristics, rhythm stability over time interval to accurately segregate a VT from Supraventricular Tachycardia (SVT) [2].
VT occurring at a rate below the detection threshold of device is termed as Slow VT. Its incidence in patients with ICD is highly variable in literature owing to the heterogeneous nature of these studies with respect to definition applied, type of ICD used, number of patients and nature of the study [3,4]. Recent studies have reported a lesser incidence 6-17% [5,6]. Although many of these episodes may be asymptomatic, they can cause syncope, palpitations, heart failure and even sudden death. More importantly, such incessant tachycardia can cause rate-induced cardiomyopathy. Advanced age, male gender and sustained monomorphic VT were independent predictors of Slow VT in the Umbrella Study [5]. However, neither NYHA functional class, underlying heart disease, rhythm (atrial fibrillation) nor concomitant resynchronisation therapy was a reliable predictor for occurrence of slow VT [6].
Programming the device to a lower TDR for detection of such episodes of Slow VT is a simple and effective method albeit at the expense of inappropriate therapies for sinus tachycardia and other supraventricular tachycardias. Such inappropriate shock delivery culminates in unnecessary painful stimuli, premature battery depletion and impairment of general well being [7,8].
The genesis of Slow VT in post ICD patients is multifactorial. Antiarrhythmic Drugs (AADs) and electrolyte imbalance are implicated but no clear causal association has been established. Although ICD is clearly superior to AAD in management of VT, adjunctive AAD is needed in up to 70% patients [9]. The primary role of AAD in such a scenario is suppression of VT’s that need appropriate therapy (ATP or Shock) by ICD. They also decrease SVT burden leading to avoidance of inappropriate therapies. As previously alluded to, the occurrence of ICD shock (irrespective of “appropriateness”) is detrimental to quality of life. However, as a collateral damage, AAD increase the VT cycle length (esp. Class I AAD and Amiodarone). They also alter the pacing and defibrillation thresholds. Some of the AAD are pro-arrhythmic too. Abnormal electrolyte milieu also alters pacing as well as defibrillation thresholds while simultaneously promoting proarrhythmia. Potassium and magnesium play a key role in electrical stability and therefore need to be monitored and corrected. Associated chronic diuretic use can cause hypokalemia and the diuretic use needs to be optimised.
With regard to ICD reprogramming and therapy, ATP usually has been shown to terminate most of the slow VT’s (>90% success rate) [10]. The “1+1” trial was a prospective trial of dual versus single chamber ICD in patients having slow VT [11]. It concluded that in dual chamber ICD, detection with a longer Tachycardia Detection Interval (TDI) improves VT detection and despite having increase in tachycardia burden it did not increase the rate of inappropriate therapies. These slow VTs could be efficiently interrupted by ATP.
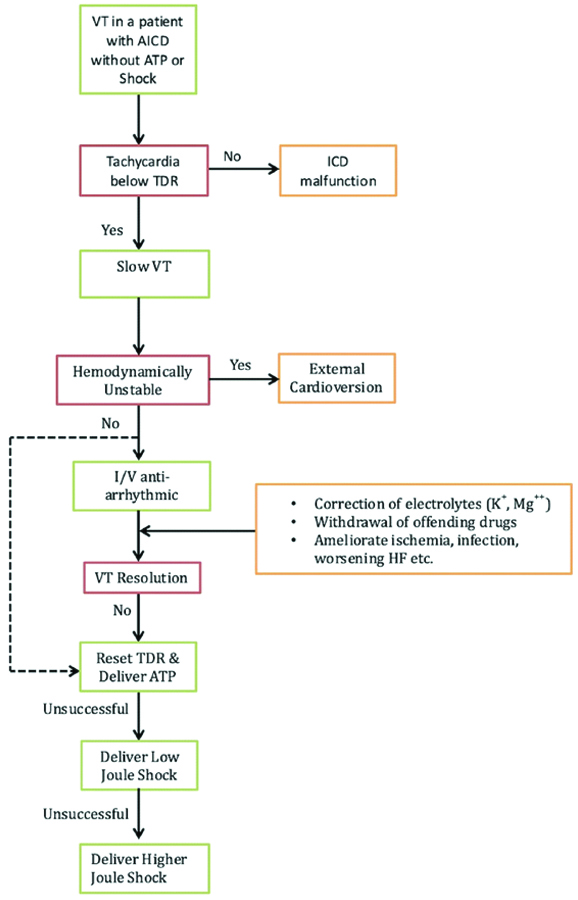
Authors describe two cases of slow VT who differed in age, duration of presentation after index event, type of ICD implanted and LVEF. However, there are many common threads like haemodynamically stable presentation, concurrent AAD use, chronic diuretic use, refractory to medical therapy as well as ATP and finally warranting ICD shocks. In both cases, the TDR was reset to detect slow VT’s and treat them with ATP. Fortunately, the events did not recur on follow-up. A suggested stepwise algorithm for managing slow VT is presented in [Table/Fig-5].
A suggested algorithm for management of Slow VT following ICD implantation.
ICD: Implantable cardioverter defibrillator; ATP: Antitachycardia pacing; TDR:Tachycardia detection rate; VT: ventricular tachycardia
Conclusion
Slow Ventricular tachycardia following Automated Implantable Cardioverter Defrillator implantation is not benign and can be quite symptomatic leading to frequent emergency department visits, in hospital admissions and rarely death. The aetiology is multifactorial and includes AADs and electrolyte imbalance. Lowering the Tachycardia Detection Rate to detect such slow VT is simple but increases the odds of delivering inappropriate shocks. Attention to correcting the electrolyte milieu and any other precipitating factors is warranted. ATPs are effective in terminating these VT successfully in most cases but not always. Unfortunately, some recalcitrant cases will need additional programmed ICD shocks.
[1]. Goldenberg I, Gillespie J, Moss AJ, Hall J, Klein H, Mc Nitt S, Long-term benefit of primary prevention with an implantable cardioverter-defibrillator: an extended 8-year follow-up study of the Multicenter Automatic Defibrillator Implantation Trial IICirculation 2010 122:1265-71.10.1161/CIRCULATIONAHA.110.94014820837894 [Google Scholar] [CrossRef] [PubMed]
[2]. Jackson LR, Daubert JP, Thomas KL, Expanding the Benefits of Implantable Cardioverter-Defibrillator Therapy: “Is Less More”?Prog Cardiovasc Dis 2012 54:372-78.10.1016/j.pcad.2011.11.00222226007 [Google Scholar] [CrossRef] [PubMed]
[3]. Bansch D, Castrucci M, Bocker D, Ventricular tachycardia’s above the initially programmed tachycardia detection interval in patients with implantable cardioverter-defibrillators: incidence, prediction and significanceJ Am Coll Cardiol 2000 36:557-65.10.1016/S0735-1097(00)00733-6 [Google Scholar] [CrossRef]
[4]. Sadoul N, Mletzko R, Anselme F, Bowes R, Schöls W, Kouakam C, Incidence and clinical relevance of slow ventricular tachycardia in implantable cardioverter-defibrillator recipients: an international multicenter prospective studyCirculation 2005 112:946-53.10.1161/CIRCULATIONAHA.105.53351316103252 [Google Scholar] [CrossRef] [PubMed]
[5]. Lusebrink U, Duncker D, Hess M, Heinrichs I, Gardiwal A, Oswald H, Clinical relevance of slow ventricular tachycardia in heart failure patients with primary prophylactic implantable cardioverter defibrillator indicationEuropace 2013 15:820-26.10.1093/europace/eus43023325044 [Google Scholar] [CrossRef] [PubMed]
[6]. Cozar-Leon R, Ruiz-Duthil AD, Perez L, Alzueta J, Martinez-Ferrer JB, Arizon JM, P Incidence and risk factors for the development of slow ventricular tachycardia in recipients of implantable cardioverter defibrillatorsEuropace 2017 19(suppl 3):iii37810.1093/ehjci/eux161.047 [Google Scholar] [CrossRef]
[7]. Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Prognostic importance of defibrillator shocks in patients with heart failureN Engl J Med 2008 359:1009-17.10.1056/NEJMoa071098 [Google Scholar] [CrossRef]
[8]. Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impactJ Am Coll Cardiol 2008 51:1357-65.10.1016/j.jacc.2007.09.07318387436 [Google Scholar] [CrossRef] [PubMed]
[9]. Henderal HV, Pinter A, Ahmad K, Korley V, Mangat I, Dorain P, Roleof anti- arrhythmic drugs in patients with implantable cardioverters and defibrillatorsEuropace 2010 12:618-25.10.1093/europace/euq07320304841 [Google Scholar] [CrossRef] [PubMed]
[10]. Wathen M, Implantable cardioverter defibrillator shock reduction using new antitachycardia pacing therapiesAm H J 2007 153:S44-S52.10.1016/j.ahj.2007.01.02017394902 [Google Scholar] [CrossRef] [PubMed]
[11]. Bänsch D, Steffgen F, Grönefeld G, Wolpert C, Böcker D, Mletzko RU, The 1+1 trial: a prospective trial of a dual- versus a single-chamber implantable defibrillator in patients with slow ventricular tachycardiasCirculation 2004 110(9):1022-29.10.1161/01.CIR.0000140259.16185.7D15326069 [Google Scholar] [CrossRef] [PubMed]