Irrigants play an important role in disinfection of root canal system thereby contributing to the success of endodontic treatment along with mechanical instrumentation. Endodontic infections are polymicrobial and frequently isolated organisms are Enterococcus faecalis and Candida albicans. E.faecalis is a gram positive facultative anaerobe and its prevalence in primary endodontic infection ranges from 4% to 40% and secondary endododontic infection ranges from 24 to 77% [1]. Kovac J et al., in his study found that cultivation of dental samples yielded four strains of E.faecalis (12.5%) and three strains of C.albicans (9.4%) [2]. Complexity of root canal system makes mechanical instrumentation inadequate to remove bacteria and tissue [3]. Therefore, irrigation is mandatory along with mechanical instrumentation to disinfect the root canal and to remove pulpal debris from root canal space [4,5].
Sodium hypochlorite (NaOCl) is considered as a gold standard irrigant used in endodontics with concentration ranging from 0.5% to 6%. It has an ability to kill most of the bacteria present in root canal space [6]. NaOCl shows both antimicrobial activity and tissue dissolving properties [7]. Since it is effective only against the organic tissue debris and bacteria, it is necessary to use with other irrigating solution for effective removal of dentin debris and smear layer [8]. The limitations of sodium hypochlorite also include unpleasant taste and toxicity. Accidental extrusion of sodium hypochlorite beyond apex causes pain, swelling, tissue necrosis and haemorrhage. Sodium hypochlorite accidents can be avoided by placing the irrigating needle short of apex, fit loosely in the canal, and the solution must be injected using a gentle flow rate [9].
Herbal extracts with least toxic effects prompted researchers to carry out more studies on its use in endodontic treatment. A study has shown that fresh minced garlic and lemon solution has inhibited growth of E.faecalis [10]. Citric acid has the property of smear removal as well as penetrates through dentinal tubules and kills microorganisms inside [11,12].
The purpose of the study was to analyse the antimicrobial activity of herbal irrigating solutions such as fresh garlic-lemon extract and ethanolic extract of garlic mixed with lemon at different time intervals (1st, 3rd and 6th day) when compared with sodium hypochlorite. The null hypothesis was that sodium hypochlorite showed increased antimicrobial efficacy when compared with ethanolic and fresh garlic-lemon extract.
Materials and Methods
The present in-vitro study was carried out in Saveetha Dental College (Saveetha Institute of Medical and Technical Sciences) after getting approval from Institutional Scientific Review Board in the month of October 2017. In our study, we have used a mixture of ethanolic extract of garlic and 100% fresh lemon juice, fresh garlic-lemon mixed in 1:1 ratio and 2.5% sodium hypochlorite.
Group 1: Ethanolic Garlic and Pure Lemon Extract Mixture
A 95% concentration garlic extract was prepared in Department of Pharmacology, Sri Ramachandra Medical College (deemed to be university). A 10 mL of prepared garlic extract was mixed with 10 mL of 100% pure lemon juice.
Group 2: Fresh Garlic-Lemon Mixture
Fresh aqueous extract of garlic was prepared by mixing 100 g of cleaned garlic bulbs and 125 mL of distilled water which was then added to a juicer and crushed. This mixture was then centrifuged at 10,000 rpm for 20 minutes and filtered using WhatManns filter paper no.1. Final concentration of garlic extract filtrate, in solution was found to be 249 mg/mL [13]. Similarly, 100% fresh lemon juice was prepared by squeezing lemon which was then filtered using WhatManns filter paper no.1. Garlic-lemon mixture was prepared by mixing 10 mL of garlic solution to10 mL of lemon solution (1:1 ratio). The prepared solution was again sterilised using syringe driven filters (Pore size-0.22 μm and Diameter 30 mm).
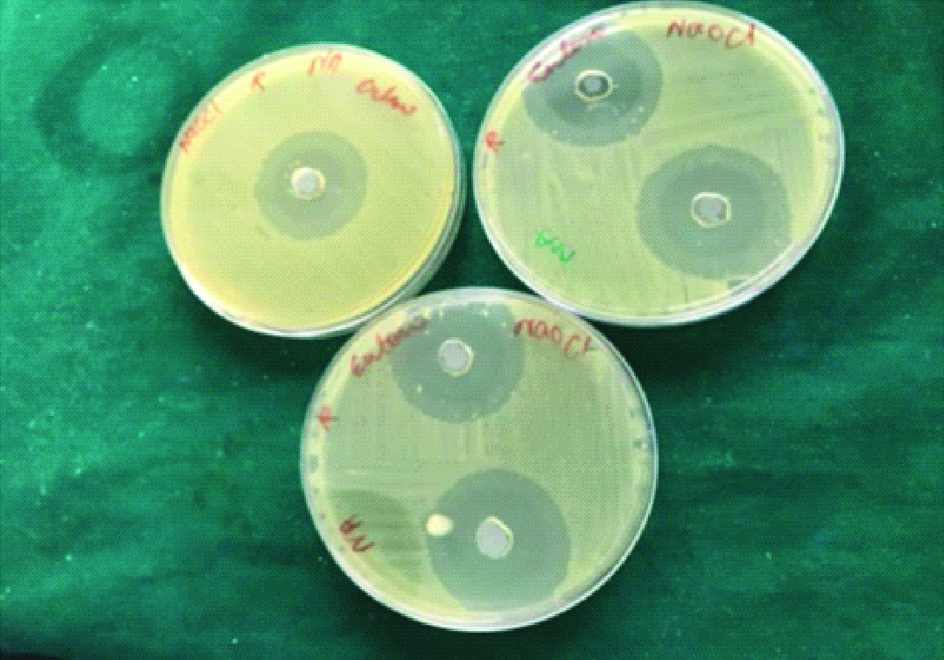
Group 3: A 2.5% Sodium hypochlorite
Commercially available 2.5% sodium hypochlorite (VENSONS INDIA) was used in this study.
The extract preparations of group 1 and 2 were made and stored in the refrigerator. Their efficacy was tested after preparing on the first day, then on the third day and the sixth day.
The antimicrobial activity was carried out using agar well diffusion method for recording zone of inhibition.
Agar well diffusion test: The standard strain of E. faecalis was inoculated on Brain Heart Infusion (BHI) agar and incubated overnight at 37°C aerobically. The purity of the culture was checked, and a suspension was made by mixing the colonies in sterile normal saline. Turbidity was then adjusted to match 0.5 Mcfarland standard.
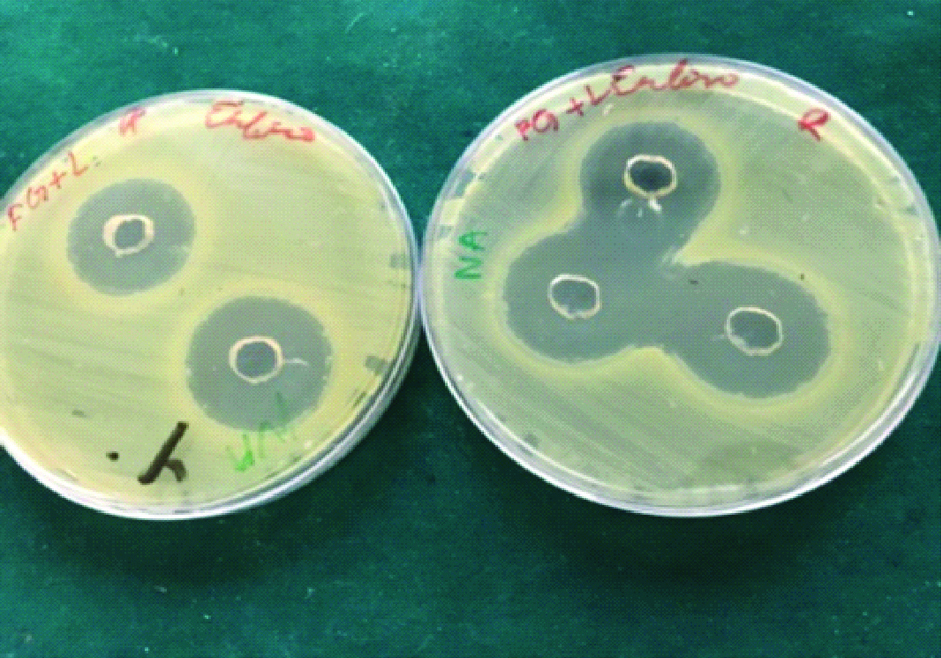
The freshly prepared BHI agar was poured into 100 mm disposable sterile petri dish. After the agar was set, a sterile metal tube of 6 mm diameter was used to cut wells on agar plates with average depth of 5 mm. The preparations of each group were placed in five wells, taking care that the preparation is well in contact with the margin. The E. faecalis suspension was spread on the agar surface using a sterile swab. Then the plates were incubated at 37°c aerobically for 24 hours. A 2.5% sodium hypochlorite was taken as a positive control. Plates were removed after incubation period and the diameter of zones of inhibition of bacterial growth attained by all three groups against the E. faecalis were recorded in millimetre and tabulated [Table/Fig-1,2 and 3].
Ethanol garlic-lemon extract.
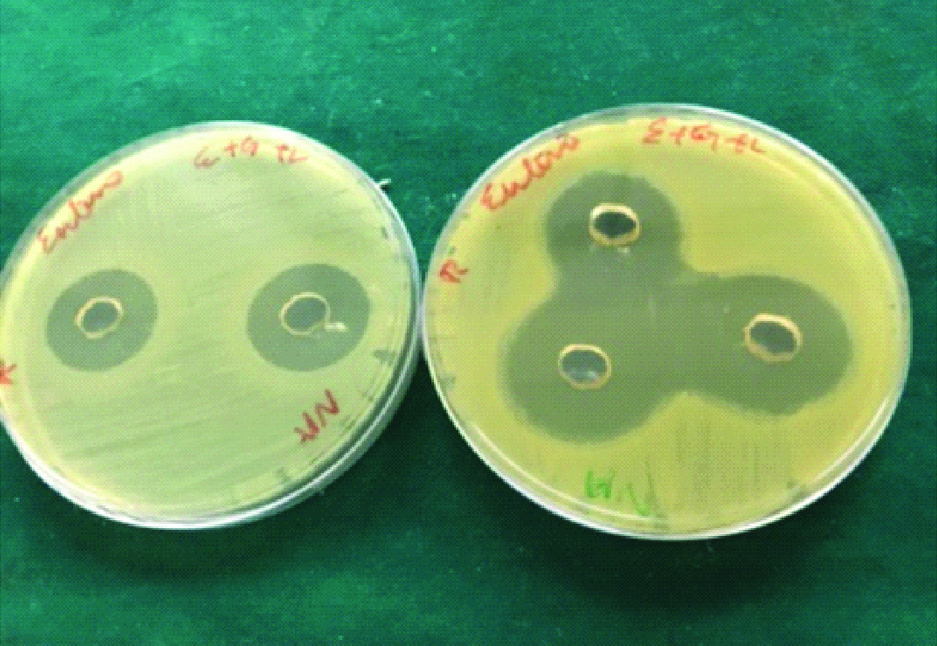
Fresh garlic-lemon extract.
Statistical Analysis
The data was analysed using Statistical Package for Social Sciences (SPSS), IBM version 23, for Windows. Statistical analysis was done using One-way ANOVA followed by post-hoc Bonferroni where p-values less than 0.05 were considered significant.
Results
The mean values and standard deviation of all the groups at day 1, 3 and 6 are depicted in [Table/Fig-4].
Showing the mean and standard deviation values of zone of inhibition (in mm) of various groups.
| Groups | | Day 1 | Day 3 | Day 6 |
|---|
| Ethanolic garlic and lemon | Mean | 24.00 | 36.80 | 34.00 |
| Standard deviation | 2.000 | 6.419 | 1.581 |
| Fresh garlic and lemon | Mean | 24.80 | 26.60 | 32.80 |
| Standard deviation | 0.837 | 1.817 | 3.271 |
| Sodium hypochlorite | Mean | 28 | 31.80 | 28.40 |
| Standard deviation | 0.000 | 1.304 | 0.548 |
[Table/Fig-5] (One-way ANOVA)) shows that there exists a significant difference between the diameters of zone of inhibition for three groups at different time intervals (first, third and sixth day).
| | Sum of squares | df | Mean square | f | Sig. |
|---|
| First day | Between groups | 44.800 | 2 | 22.400 | 14.298 | 0.001 |
| Within groups | 18.800 | 12 | 1.567 | | |
| Total | 63.600 | 14 | | | |
| Third day | Between groups | 260.133 | 2 | 130.067 | 8.446 | 0.005 |
| Within groups | 184.800 | 12 | 15.400 | | |
| Total | 444.933 | 14 | | | |
| Sixth day | Between groups | 86.933 | 2 | 43.467 | 9.659 | 0.003 |
| Within groups | 54.400 | 12 | 4.500 | | |
| Total | 140.933 | 14 | | | |
On first day, group 3 showed statistically significant maximum inhibitory effect with zone of inhibition (28 mm, mean) when compared to group 1 and group 2. The zone of inhibition of both ethanolic garlic-lemon mixture and fresh garlic-lemon extract was found to be 24.00 mm and 24.80 mm mean, respectively.
When tested on third day, group 1 showed statistically significant maximum inhibitory effect when compared with group 3 and 2. The zone of inhibition of ethanolic garlic-lemon mixture (36.80 mm) was more when compared with sodium hypochlorite (31.80 mm) and fresh garlic-lemon mixture (26.60 mm).
On sixth day, group 1 showed statistically significant inhibitory effect followed by group 2 and group 3. The zone of inhibition of group 1, 2 and 3 were found to be 34 mm, 32.80 mm and 28.40 mm respectively.
[Table/Fig-6] show post-hoc Bonferroni test for inter comparison of the antimicrobial efficacy of different groups against E. faecalis. On first day, post-hoc revealed that there was statistically significant difference between all groups, except between ethanolic extract of garlic-lemon and fresh garlic-lemon, and vice versa. On third day, post-hoc revealed there was statistically significant difference only between ethanolic garlic-lemon and fresh garlic-lemon, and vice versa. On sixth day, post-hoc revealed that there was statistically significant difference between all groups, except ethanolic extract of garlic-lemon and fresh garlic-lemon, and vice versa.
| 0 | Groups | Mean difference | Std. error | Sig. | 95% Confidence interval | 95% Confidence interval |
|---|
| Groups | Lower bound | Upper bound |
|---|
| First day | Ethanolic garlic-lemon | Fresh garlic-lemon | -0.800 | 0.792 | 0.996 | -3.00 | 1.40 |
| Sodium hypochlorite | -4.000* | 0.792 | 0.001 | -6.20 | -1.80 |
| Fresh garlic-lemon | Ethanolic garlic-lemon | 0.800 | 0.792 | 0.996 | -1.40 | 3.00 |
| Sodium hypochlorite | -3.200* | 0.792 | 0.005 | -5.40 | -1.00 |
| Sodium hypochlorite | Ethanolic garlic-lemon | 4.000* | 0.792 | 0.001 | 1.80 | 6.20 |
| Fresh garlic-lemon | 3.200* | 0.792 | 0.005 | 1.00 | 5.40 |
| Third day | Ethanolic garlic-lemon | Fresh garlic-lemon | 10.200* | 2.482 | 0.004 | 3.30 | 17.10 |
| Sodium hypochlorite | 5.000 | 2.482 | 0.201 | -1.90 | 11.90 |
| Fresh garlic-lemon | Ethanolic garlic-lemon | -10.200* | 2.482 | 0.004 | -17.10 | -3.30 |
| Sodium hypochlorite | -5.200 | 2.482 | 0.174 | -12.10 | 1.70 |
| Sodium hypochlorite | Ethanolic garlic-lemon | -5.000 | 2.482 | 0.201 | -11.90 | 1.90 |
| Fresh garlic-lemon | 5.200 | 2.482 | 0.174 | -1.70 | 12.10 |
| Sixth day | Ethanolic garlic-lemon | Fresh garlic-lemon | 1.200 | 1.342 | 1.000 | -2.53 | 4.93 |
| Sodium hypochlorite | 5.600* | 1.342 | 0.004 | 1.87 | 9.33 |
| Fresh garlic-lemon | Ethanolic garlic-lemon | -1/200 | 1.342 | 1.000 | -4.93 | 2.53 |
| Sodium hypochlorite | 4.400* | 1.342 | 0.020 | .67 | 8.13 |
| Sodium hypochlorite | Ethanolic garlic-lemon | -5.600* | 1.342 | 0.004 | -9.13 | -1.87 |
| Fresh garlic lemon | -4.400* | 1.342 | 0.020 | -8.13 | -0.67 |
Discussion
Root canal therapy aims to eradicate the microbes from the root canal system and to prevent the secondary infections after treatment. Mechanical debridement becomes difficult due to anatomical complexities like lateral or furcal canals, fins, webs, apical deltas and isthmus. Therefore, endodontic irrigant solutions are necessary to complement the mechanical action thereby facilitating maximum removal of microorganisms [14].
Sodium hypochlorite in concentrations ranging from 0.5 to 6% has been advocated for irrigation of root canal space. It has tissue dissolving and antimicrobial properties. Antimicrobial activity of sodium hypochlorite is based on its high pH and presence of chlorine. The high pH of sodium hypochlorite interferes with the integrity of cytoplasmic membrane, biosynthetic alterations in cellular metabolism, and phospholipid degradation [15]. Chlorine predominantly exists as hypochlorous acid (HOCL) at acidic and neutral pH whereas at high pH of 9 and above hypochlorite ion predominates. Antibacterial activity of NaOCl is mainly by hypochlorous acid [16].
The main active component of garlic is allicin which destroys the cell wall and cell membrane of root canal bacteria and thus can be used as an irrigant alternative to sodium hypochlorite [17]. Allicin has ability to prevent both germination of spores and growth of hyphae. Concentrated garlic extract (95%) contains 34% allicin, 44% total thiosulfinates, and 20% vinyldithiins which is believed to be responsible for antimicrobial activity [18]. It has shown that 1 mg of allicin is equated to that of 15 IU of penicillin [19]. It is bactericidal and has shown antimicrobial activity against wide variety of bacterial species such as E.coli, S.aureus B.cereus, Salmonella, listeria, Proteus and Streptococcal species [20,21]. The main antimicrobial effect of allicin is due to its chemical reaction with thiol groups of various enzymes [22]. One of the limitations of garlic is its pungent smell which can be overcome by using flavonoids [23]. Further research should be done on interaction of flavonoids with garlic.
Lemon juice (pH 2.21) is a natural source of citric acid (pH 1.68) with lower acidity. Citric acid being a chemical product has some irritating effect to periapical tissues when compared to natural lemon solution. Lemon has antioxidant, antiviral, antibacterial, antifungal and anti-cancerous activity [24]. It has ascorbic acid, phenolic acids, polyphenols and dietary fibers as its components [25]. The fresh lemon solution has been used as a root canal medicament due to its wide antibacterial efficiency including E.faecalis [26]. It also showed antimicrobial activity against S.aureus, Klebsiella, E.coli, P.aeruginosa, C.albicans, S.aureus and S.pneumoniae which is in support to our study [27,28].
No studies have reported till date about the combination of garlic-lemon mixture as a root canal irrigant. In our study, ethanolic garlic-lemon mixture has showed enhanced antimicrobial efficacy followed by fresh garlic-lemon when compared with sodium hypochlorite which indicates that antimicrobial activity of garlic lemon mixture has increased with time. Lemon in garlic-lemon mixture helped in masking smell of garlic by inhibiting volatile sulphide compound production [29]. This combination can become potential irrigant in endodontics as lemon extracts helps in removing smear layer thereby killing bacteria inside the dentinal tubules [30].
In our study, syringe driven filters are used thereby maintaining the sterility of solution as normal microorganism such as bacteria (bacterial size ranges from 1 μm to about 5 μm) which cannot pass through the pores of filters.
NaOCl had statistically significant superior antibacterial activity against E.faecalis than all other groups on first day. But on third and sixth day ethanolic extract of garlic-lemon has showed significant antibacterial effect when compared with fresh garlic lemon and sodium hypochlorite. The null hypothesis was rejected as garlic-lemon mixture has showed increased antimicrobial efficacy when compared with sodium hypochlorite.
Limitation
However, till date no literature have reported the antimicrobial efficacy of combination of herbal extracts like garlic and lemon, the present study has following limitations:
1) Antimicrobial efficacy was tested only against E. faecalis where endodontic infections are polymicrobial. Further studies should be carried out with other microorganisms.
2) Clinical effectiveness of garlic-lemon combination might vary in in-vivo studies. The effect of garlic-lemon should be checked clinically.
3) Tissue dissolving capacity of garlic-lemon was not checked whereas, it is significant in sodium hypochlorite, and considered to be a gold standard irrigant in endodontics.
Conclusion
With in the limitations of this study, garlic-lemon solution (fresh and ethanolic extract) showed increased antimicrobial efficacy at the end of sixth day when compared to sodium hypochlorite. Ethanolic extract had shown maximum inhibitory effect compared to fresh garlic-lemon and sodium hypochlorite. The garlic-lemon solution can be a potential alternate as irrigant in endodontics in near future.