Breast cancer is the most commonly diagnosed cancer in women worldwide with over 2.1 million new cases per year accounting for almost one in four cancer cases among women [1]. There are geographic and ethic variations in the incidence and mortality due to breast cancer. Although, these may be explained by hereditary and genetic factors like family history of breast cancer or inherited mutations, studies of migrants have shown that non-hereditary factors are the major drivers of the observed international and inter-ethnic differences in incidence. The development of a malignancy and its progression is a complex interplay of genetic abnormalities, environmental factors and host interactions of the tumour cells. One such important but still poorly understood non-hereditary factor in the development and prognosis of a tumour is the role of host immune system. Since the initial reports of TILs over 40-years-ago, their role in the pathobiology is still being debated [2,3]. Initial views that high TILs represent anti-tumour immunity and translate to good prognosis were found to be overly simplistic. While TILs represent anti-tumour immune response, the outcome depends on many factors like the presence of antigen presenting cells, immunostimulatory cytokines, the subsets of lymphocytes involved, optimal surface molecule expression by both the tumour cells and the infiltrating lymphocytes etc. The complexities of these interactions are just beginning to be defined and various forms of cancer appear to have different immune system requirements [3]. Previous studies have shown that high TILs at diagnosis are associated with a higher pathological complete response after neoadjuvant chemotherapy [4]. Similarly higher baseline TILs in some randomised controlled trials were associated with high proliferative index, higher grade and Oestrogen Receptor (ER)- negative tumours and represent a strong prognostic factor for certain breast cancer subtypes, especially the Triple-Negative Breast Cancer (TNBC) [5,6]. Currently, TIL estimation is not part of the pathology reporting protocols for breast carcinoma and the role of TIL in the prognosis of untreated patients is unknown. The present study evaluates TILs in Indian breast carcinoma patients and their correlation to known prognostic factors and outcomes in patients of early breast carcinoma who have undergone definitive surgical intervention with curative intent.
Materials and Methods
A retrospective and prospective Cohort study was carried out at a large tertiary care oncology referral centre of Armed Forces Medical College, Pune, Maharashtra, India. Seventy-five patients of early breast carcinoma who underwent definitive surgical excision of the tumour in the form of Modified Radical Mastectomy (MRM) or Breast Conservation Surgery (BCS) between January 2014 to November 2017 were included. The inclusion criteria were Histopathological Examination (HPE) proven cases of Breast carcinoma who have undergone definitive surgery for breast carcinoma with information on pathological grade and TNM stage of disease. Patients with secondary malignant lesions of breast, incomplete clinical, prognostic and follow-up information and those exhibiting neo-adjuvant chemotherapy prior to surgery were excluded. Prior approval of the Institutional Ethics Committee was obtained vide AFMC IEC/141/2017 dt 28 Sep 2017.
For all cases, the HPE slides were re-examined for the grade, stage and tumour type and one representative FFPE block with predominantly viable tumour tissue identified for IHC. Tumours were graded by Nottingham modification of the Bloom-Richardson system [7] and staged using the TNM system as per the 8th edition of American Joint Committee on Cancer (AJCC) staging manual [8]. The cases were categorised into molecular subtypes as Luminal A (ER + and/or PR+, Ki67 < 20% and HER2-), Luminal B (ER + and/or PR+, Ki67 > 20% and/or HER2+), HER2-positive (ER-, PR- and HER2+) and Triple-negative type (ER-, PR-, HER2-) according to the St Gallen International Breast Cancer Consensus criteria of 2013 [9].
Immunohistochemistry
IHC staining was carried out in all 75 cases for ER (Monoclonal mouse Antihuman Oestrogen receptor; Clone 1D5, RTU, DakoCytomaton Inc, Denmark), PR (Monoclonal mouse Antihuman Progesterone receptor; Clone PgR 636, RTU, DakoCytomatonInc, Denmark), Her2/neu (Polyclonal rabbit antihuman c-erbB-2 oncoprotein; DakoCytomatonInc, Denmark), Ki67 (Monoclonal Mouse Antihuman Ki67 Antigen Clone MIB-1, Dako CytomatonInc, Denmark) and CD8 (Monoclonal Mouse Antihuman CD8 Antigen, Clone C8/144B, DakoCytomatonInc, Denmark) was carried out. Visualisation was done using the EnVision+ Dual Link system-polymer HRP IHC detection system (DakoCytomatonInc, Denmark). Appropriate positive controls (endometrium for ER/PR; known strongly positive, Fluorescent InSitu Hybridisation confirmed case of breast carcinoma for Her 2/Neu; tonsils with reactive hyperplasia for Ki-67, CD8) and negative controls (Positive controls with the primary antibody omitted) were incorporated in each run.
Scoring Systems for IHC Expression
Expression of ER and PR was scored as per Allred scoring system [10] and Her2/neu expression was done as per College of Anatomic Pathologists (CAP) protocol. Ki67 proliferation index was calculated by the number of positively stained nuclei to total nuclei in a 500 cell count. Ki67 labelling index of less than or equal to 20% was considered the cut-off for differentiating Luminal subtype A and B.
CD8 T-Cell Counts
Scoring for CD8 expression was done based on modification of the previously described method by Rathore AS et al., [11]. In brief, all CD8 stained slides were blinded and randomised by one researcher (NG) and five random areas from each slide were photographed by a second blinded pathologist (DKR). The images were further shuffled and numbered by the first author researcher (NG). These coded images were reviewed independently by two researchers (DKR, SK). All CD8 positive cells were counted in each of the images. TILs were further classified as Intra-tumoural Tumour infiltrating Lymphocytes (iTILs) and Stromal Tumour infiltrating Lymphocytes (sTILs). iTILs were defined as CD8+ lymphocytes located within tumour cell nests or in direct contact with the breast carcinoma malignant epithelial cells, whereas sTILs were defined as CD8+ lymphocytes in the adjacent peritumoral stroma without direct contact with the carcinoma cells. Total CD8+ TILs (tTILs) were measured by combining the counts of iTILs and sTILs for each image. The mean TIL values from all the images counted for each case was used for further comparisons. All counts with more than 5% variation between the two researchers were resolved by a repeat consultative counting.
Survival Data
Patients records, as well as a telephonic survey were used to ascertain the current status of the patients. A telephonic informed consent to provide survival information was obtained at the time of the telephonic survey. Overall Survival (OS) was defined as interval between the initial diagnosis and death while Disease Free Survival (DFS) was defined as the period from the date of definitive surgery to the date of relapse.
Statistical Analysis
The categorical variables are summarised by proportion. The continuous/discrete variables are summarised by median and inter quartile range and Wilcoxon-Mann-Whitney test was used for their comparison across different groups. A significance threshold of 0.05 was used. R software ver 3.2 was used for statistical analysis.
Results
On scrutiny of patient HPE and clinical records, 122 of the 184 cases of biopsy-proven breast carcinoma who had undergone definitive surgery for breast carcinoma were identified. Of these, eight were rejected due to non-availability of FFPE blocks, 27 due to prior neoadjuvant chemotherapy, nine due to inadequate clinical or follow-up information and three patients declined consent to participate in the study when contacted telephonically. The remaining 75 cases were included in the study. The study subjects had a mean age of 52.69±12.07 with a range of 30-78 years.
Of the 75 cases, 16% (n=12) were well differentiated tumours (Grade 1), 56% (n=42) were moderately differentiated (grade 2) and 28% (n=21) were high grade tumours (grade 3). An overwhelming majority of the tumours were in TNM stage II (63%; n=47). The follow-up period of the patients ranged from 3 months to 39 months with a median of 548 days. There were only three deaths during the period with two of them being unrelated to breast carcinoma. Hence, there was insufficient data for overall survival. However, there were eight patients who had relapse of the disease process (11%) with a DFS of 116 to 903 days and a median of 388 days. The general patient characteristics are summarised in [Table/Fig-1].
General patient characteristics.
| Characteristics | Number (%) |
|---|
| Age |
| Mean (SD) | 52.69 (12.07) |
| Range | 30-78 years |
| Tumour grade (modified bloom-richardsons grade) |
| Grade 1 (Low) | 12 (16%) |
| Grade 2 (Intermediate) | 42 (56%) |
| Grade 3 (High) | 21 (28%) |
| Tumour size (AJCC 8th edition) |
| T1 (<2.0 cm) | 9 (12%) |
| T2 (2.0-5.0 cm) | 52 (69%) |
| T3 (>5.0 cm) | 11 (15%) |
| T4 (Extension to skin/Chest wall) | 3 (04%) |
| Regional lymph node status (AJCC 8th edition) |
| N0 (No nodes involved) | 37 (50%) |
| N1 (1-3 axillary involved) | 19 (25%) |
| N2 (3-9 axillary/internal mammary nodes involved) | 12 (16%) |
| N3 (>10 axillary nodes/infraclavicular nodes involved) | 7 (09%) |
| Overall TNM stage (AJCC 8th edition) |
| Stage I A | 7 (09%) |
| Stage IB | 0 (0%) |
| Stage II A | 29 (39%) |
| Stage II B | 18 (24%) |
| Stage III A | 12 (16%) |
| Stage III B | 2 (03%) |
| Stage III C | 7 (09%) |
| Relapse status |
| Yes | 8 (11%) |
| No | 67 (89%) |
IHC examination revealed 63% (n=47) cases positive for ER expression, 47% (n=35) positive for PR expression, 29% (n=22) positive for Her 2 Neu and 65% (n= 49) showing High Ki67 index. Representative positive cases of IHC markers used are given as [Table/Fig-2]. The molecular subtype classified as per St Gallen Consensus criteria were predominantly of the luminal type (63%; n=47) with the HR and the TNBC groups being 14% (n=11) and 23% (n=17) respectively. The distribution of IHC markers and Molecular grades are given in [Table/Fig-3].
IHC panel for classification of breast carcinoma.
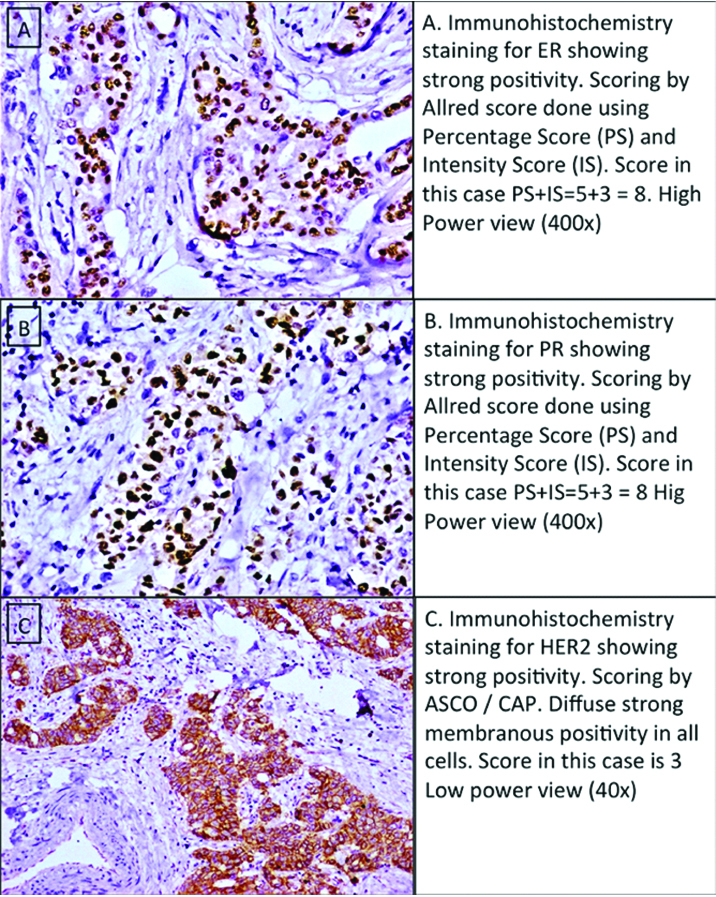
IHC marker status and molecular subtypes.
| Characteristics | Number (%) |
|---|
| IHC marker status |
| ER Positive | 47 (63%) |
| ER Negative | 28 (37%) |
| PR Positive | 35 (47%) |
| PR Negative | 40 (53%) |
| Her2 Positive | 22 (29%) |
| Her2 Negative | 53 (71%) |
| Ki67 Low (<20%) | 26 (35%) |
| Ki67 High (>20%) | 49 (65%) |
| Molecular subtypes(by surrogate ihc markers as per st gallen consensus criteria) |
| Luminal A | 17 (23%) |
| Luminal B | 30 (40%) |
| Her 2 Enriched | 11 (14%) |
| TNBC | 17 (23%) |
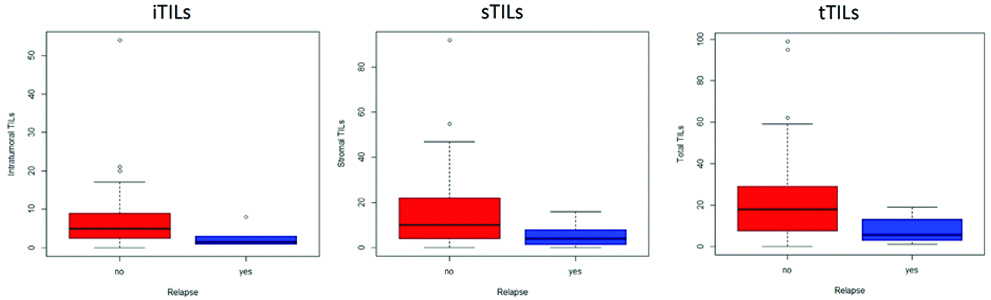
For evaluation of CD8+ cytotoxic T Cells and the association of TILS with the various molecular groups and relapse, median with Inter Quartile Range (IQR) was used along with non-parametric tests, the details of which are summarised in [Table/Fig-4]. The median TILs was 4 (2,9), the median sTILs was 9 (3,18.5) and the median tTILs was 16 (6,28). Representative photomicrographs of CD 8 stained slides are given as [Table/Fig-5,6 and 7]. A statistically significant association was seen with iTIL (p=0.041) and tTIL (p=0.037) for patients with relapse as lower TIL counts were associated with relapse. A similar trend was also seen with sTILs, however, the difference was not statistically significant. The association of TILs to relapse status is shown in [Table/Fig-8]. Due to the short follow-up period and small number of cases with relapse and death, a survival analysis or Kaplan-Meyer curves could not be made. No association, however, was seen with hormonal receptor status, Her2 Neu positivity, the intrinsic molecular subtypes or other known prognostic markers like the grade, tumour size, nodal metastasis or TNM stage.
Comparison of iTIL, sTIL and total TIL scores.
| Characteristics | iTIL | sTIL | tTIL |
|---|
| Median (IQR) | p-value | Median (IQR) | p-value | Median (IQR) | p-value |
|---|
| Oestrogen receptor status |
| Positive | 4 (1-9) | 0.403 | 9 (2-15) | 0.653 | 16 (5-27) | 0.591 |
| Negative | 4.5 (3-11.25) | | 9 (5-22.5) | | 17 (7-29) | |
| Progestogen receptor status |
| Positive | 3 (1-8) | 0.066 | 8 (2-14) | 0.115 | 10 (4-27) | 0.078 |
| Negative | 6 (3-10.5) | | 10.5 (5-23) | | 19 (10-30.5) | |
| Her 2 Neu Status |
| Positive | 6.5 (1.75-12) | 0.332 | 12 (7.25-19.25) | 0.398 | 19.5 (9-28.75) | 0.405 |
| Negative | 4 (1.5-8) | | 9 (2.5-20) | | 12 (5-28.5) | |
| Ki-67 Status |
| <14 | 3.5 (1.75-6.25) | 0.217 | 8 (3-18.5) | 0.78 | 11.5 (5.5-23.75) | 0.507 |
| >14 | 6 (1.5-10.5) | | 10 (2-20) | | 18 (5.5-29) | |
| Intrinsic molecular subtype |
| Luminal | 4 (1-9) | 0.633 | 9 (2-15) | 0.39 | 16 (5-27) | 0.59 |
| HR | 4 (3-12) | | 16 (8-23) | | 21 (15-28) | |
| TNBC | 5 (2-10.5) | | 6 (2.5-22) | | 11 (4.5-33) | |
| Tumour grade |
| Grade 1 | 3.5 (0.75-8.25) | 0.441 | 9 (2.25-21) | 0.835 | 15 (3-31.5) | 0.784 |
| Grade 2 | 3.5 (1-9.5) | | 9 (3.75-16) | | 16.5 (6-27.25) | |
| Grade 3 | 7 (3-8) | | 12 (2-26) | | 19 (5.5-34) | |
| Tumour size |
| T1 lesions | 4 (0.5-12.5) | 0.844 | 14 (1-24.5) | 0.769 | 27 (1.5-28) | 0.896 |
| All other T | 4 (2-9) | | 9 (3-18.25) | | 16.5 (6-29) | |
| Lymph node status |
| Node Negative | 4 (1-9) | 0.774 | 10 (2-27) | 0.461 | 19 (4-34.5) | 0.348 |
| Node Positive | 4 (2.75-9) | | 9 (3.75-14.5) | | 13.5 (6.75-23.75) | |
| Stage of disease |
| Early Breast Carcinoma (TNM Stage I+II) | 4 (1-9) | 0.799 | 9 (2-23) | 0.962 | 17.5 (4.75-31.25) | 0.759 |
| Advanced Breast Carcinoma (TNM Stage III+IV) | 4 (3-8) | | 10 (4.5-13.5) | | 16 (8-23.5) | |
| Relapse |
| Yes | 5 (2-9) | 0.041 | 10 (4-23) | 0.071 | 18 (7-29) | 0.037 |
| No | 1.5 (1-3) | | 4 (1.25-9) | | 5.5 (2.5-13.5) | |
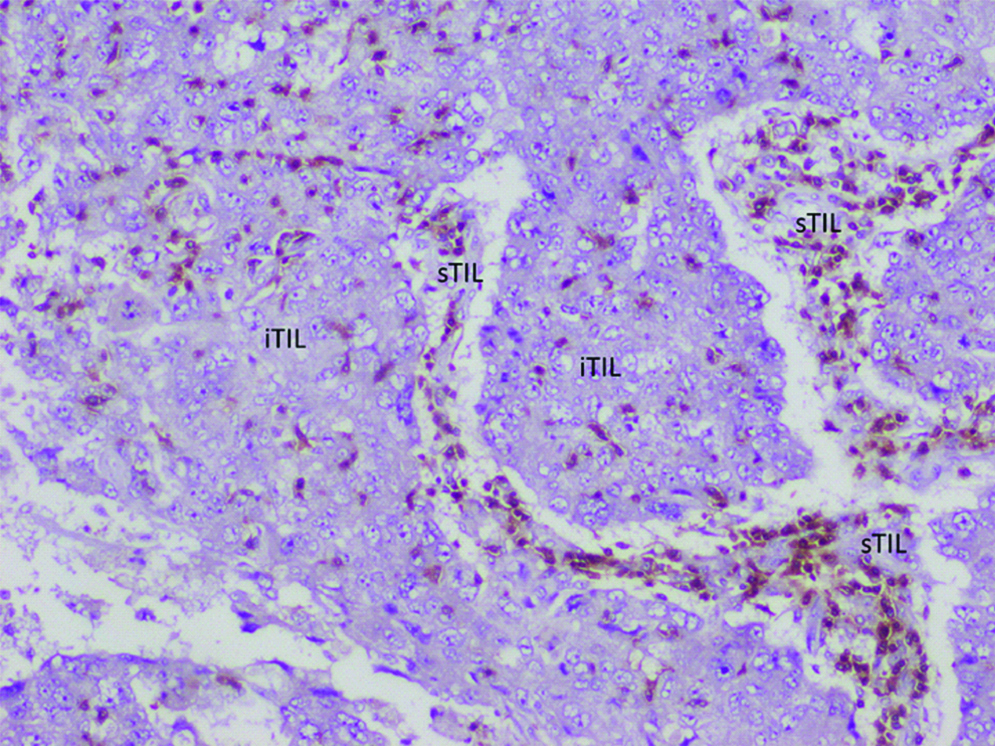
IHC Stained section showing the brown stained CD 8+ T cells infiltrating breast carcinoma cells. The T cells can be seen infiltrating between the epithelial tumour cells (iTILs) as well as in the intervening stroma (sTILs).
IHC for CD8; counterstain Haematoxylin; 400x
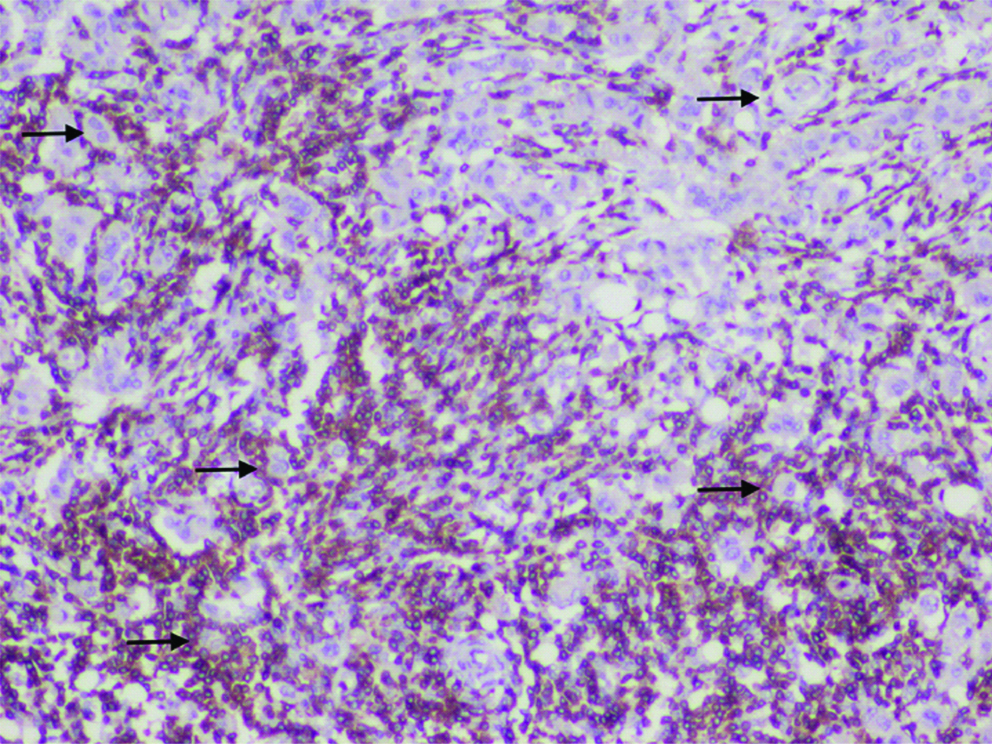
IHC Stained section showing intense infiltration by iTILs with many individual tumour cells surrounded by CD8+ cells (Arrows).
IHC for CD8; counterstain Haematoxylin; 400x
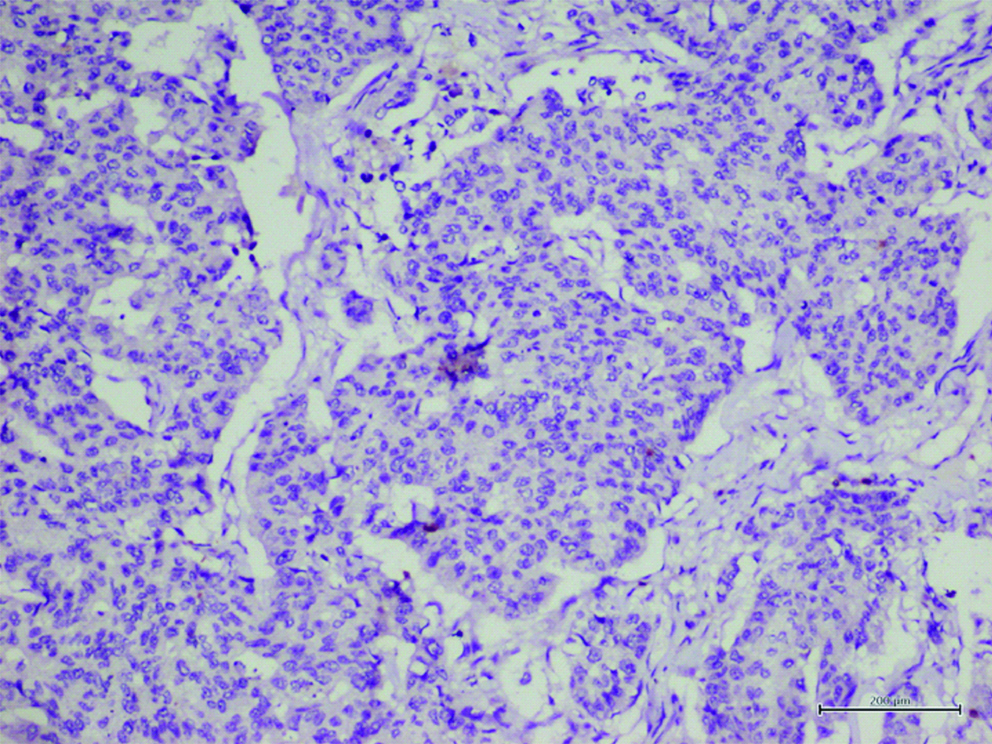
IHC Stained section that is negative for infiltration by TILs. Both sTILs and iTILs are absent.
IHC for CD8; counterstain Haematoxylin; 400x
Association of TILs with relapse status of patients.
Discussion
TILs have been described in most types of solid tumours, including breast cancer, colon cancer, cervical cancer, melanoma and lung cancer [12,13]. The presence of TILs in breast cancer represents the host response to the tumour tissue indicative of anti-tumour immunity [14] and a positive association between higher numbers of TILs with good prognosis has been documented as early as 1922 [15]. However, the association between TIL and prognosis is not straightforward and has been the subject of intense research and discussion. While a number of studies have contributed to understanding the function and origins of TILs and the relationship between higher levels of TILs and improved prognosis in patients with early stage breast cancer has now been confirmed in thousands of patients [15-17], there are many studies that report apparently contradictory findings [18]. Alkatout I et al., reported that expression of CD3+ and CD8+ lymphocytes had no statistically significant impact on disease-free and overall survival in invasive ductal breast cancer while Matkowski R et al., observed that patients with high expression of CD 4+ and CD8+ TILs had distinctly worse cancer specific overall survival [18,19]. Among Indian patients, Rathore AS et al., have shown a favourable prognosis in those with CD 4+ and CD 8+ TILs [11]. In the present Cohort, authors did find a positive association between fewer CD8+ iTIL and tTIL and earlier relapse. A similar trend was also noted with sTIL although it was not statistically significant (p=0.071). Authors found that fewer iTILs and tTILs but not sTILs are associated with earlier relapse. However, the short follow-up period in the present study may be an inherent bias and longer follow-up of the same cohort may result in a stronger and clearer association. Further in evaluating TILs in invasive breast cancer, it is not just the quantity and distribution of the TIL subsets that determined the outcome but the complex tumour immune microenvironment and interactions between the multiple lymphocyte subtypes that may play a role in the outcome of breast cancer recurrence [20].
Similarly, there is contradicting outcomes reported in literature regarding the association between CD8+ TIL and conventional prognostic factors like grade, stage and lymph node status in breast carcinoma. Kim ST et al., reported that decreased number of CD8+ TILs in breast tumours were significantly associated with lymph node metastasis, higher stage and high proliferative index [21]. However, La Rocca G et al., observed that the number of CD4 and CD8 expressing cells was higher in node negative than in node positive invasive ductal breast lesions [22] while Georgiannos SN et al., reported that the CD8+ infiltrate was more intense and more activated in node positive tumours that have worse prognostic indices [23]. In the present study, there was no association between TILs and conventional prognostic markers like grade, stage, tumour size and nodal status of the tumour. This highlights the heterogeneity and the complex interactions at play between the TILs and the tumour tissue. In a large study, Baker K et al., showed that women with high lymphocyte count and ER-positive tumours had an inferior out come but only if tumours were also of low-grade and a superior outcome in ER-negative tumours only if tumours were also of high grade [24]. Hence large studies of more homogenous groups are still required to better understand the complex interplay between the TILs and the overall outcome of individual patients.
Tumour-infiltrating lymphocytes appear to be associated with clinicopathological factors such as negative steroid receptor status and HER2 overexpression [25]. In one of the largest studied cohorts of women with lymph-node-positive disease, the median percentage of stromal tissue infiltrated with TILs was 10% in ER-positive and HER2-negative samples, 15% in HER2-positive samples, and 20% in ER-negative and HER2-negative disease [5]. Although a prognostic association between stromal TILs and ER-positive and HER2-negative early stage breast cancer has not been found, expression of immune-related genes in this subtype is associated with a better prognosis [26]. Evaluation of TILs with respect to the intrinsic molecular subtypes and evidence of positive prognostic impact of both CD3 and CD8 markers have been demonstrated in Triple-Negative Breast Cancer (TNBC) [5,27,28]. There was no such association between the various molecular subtypes and TILs in this study. Although authors did find Her2 enriched type showing much higher median value of sTIL than iTIL, the same was not statistically significant. These differences may be inherent to the Indian population as other workers in India and Kenya have also reported a similar lack of association of TILs with the intrinsic molecular subtypes [29-31].
The present study shows that even in a short follow-up period of just around three years, there is a statistically significant association between TILs and the incidence of relapse in a mixed population of breast carcinoma patients. There is increasing evidence that the magnitude and composition of tumour immune infiltrate can affect prognosis and response to therapy in invasive breast cancer and therefore there is scope for the use of pre-therapy tumour immune environment as both a biomarker for the prognosis as well as a guide to determine the ideal therapy. Currently, the International TILs Working Group has standardised the evaluation of breast cancer TILs for use in clinical trials as well as routine clinical practice [32]. Standardising the characterisation of breast TILs by both the subtype and immune environment will allow the identification of patients for the various emerging immune therapies and highlights the need for more large scale studies and meta-analysis of available data to make sense of the complex interactions of the host immune system and the tumour microenvironment.
Limitation
This study has its limitations in the small sample size and short follow-up period. Inadequate numbers in the subgroups may be responsible for the lack of association between the known prognostic factors like tumour size, proliferation index, nodal status and TILs. Therefore, larger studies with longer prospective follow-up of the cohorts would be required for validating the findings in this study. Further in order to understand the complex interactions of various cytotoxic, regulatory and helper T cell populations and the tumour microenvironments more focussed subgroups with analysis of the various T cell subgroups would be the way forward. The lack of association between TILs and molecular subtypes in Indian patients also needs to be further evaluated.
Conclusion
The study shows that there is a significant association between the iTIL and tTIL but not sTIL with relapse of the disease in Breast carcinoma. Patients with a higher TIL are likely to have a better prognosis and fewer relapses. There was however no association between TILs and hormonal receptor status, Her2 neu positivity, the intrinsic molecular subtypes or other known prognostic markers like the grade, tumour size, nodal metastasis, or TNM stage.