Bloodstream Infection Caused by Candida auris: A Case Report of an Emerging Fungal Infection–Misidentified by VITEK2
Rup Jyoti Chandak1, Bibhabati Mishra2, Poonam Sood Loomba3
1 Ex-Senior Resident, Department of Microbiology, Gobind Ballabh Pant Institute of Postgraduate Medical Education and Research, New Delhi, India.
2 Director, Professor, Department of Microbiology, Gobind Ballabh Pant Institute of Postgraduate Medical Education and Research, New Delhi, India.
3 Director, Professor, Department of Microbiology, Gobind Ballabh Pant Institute of Postgraduate Medical Education and Research, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Bibhabati Mishra, Room Number 301, 3rd Floor, Academic Block, Gate Number 2, Gobind Ballabh Pallabh Institute of Postgraduate Medical Education and Research, New Delhi-110002, India.
E-mail: bibham89@yahoo.com
This report describes nosocomial blood stream infection caused by Candida auris in a patient with neurological illness in a tertiary care hospital. Blood culture was performed by Bactec and initial identification and sensitivity by VITEK 2. It was misidentified as Candida haemulonii with sensitivity to Caspofungin, Micafungin and Voricanazole. MALDI-TOF analysis on the isolate confirmed it as Candida auris. However, patient was treated with Caspofungin and showed clinical improvement. Thus, multiple automated methods should be employed to accurately diagnose bugs causing life threatening infections.
Automated, Caspofungin, Nosocomial, Voricanazole
Case Report
A 24-year-old previously healthy male patient presented to the neurology outpatient department of a tertiary care hospital with chief complaint of acute onset behaviour change with headache followed by weakness of all the four limbs along with tingling and numbness with slurred speech. There were occasional episodes of seizure. His past medical history was not significant. Magnetic Resonance Imaging (MRI) of the brain showed white matter and corpus callosum hyperdense region. He was normotensive and non-diabetic. Cerebrospinal Fluid (CSF) cytology showed normal cell count, sugar and protein and sterile on aerobic culture. Patient was diagnosed as Acute Disseminated Encephalomyelitis (ADEM) and neuroleptic malignant syndrome {NMS (post-resperidone)} and started on by injectable steroids. His clinical conditions deteriorated and he was mechanically ventilated. After 45 days of hospital stay, patient developed fever (100°F) with raised total leucocyte counts (16000/cc).
Microbiological Work-Up
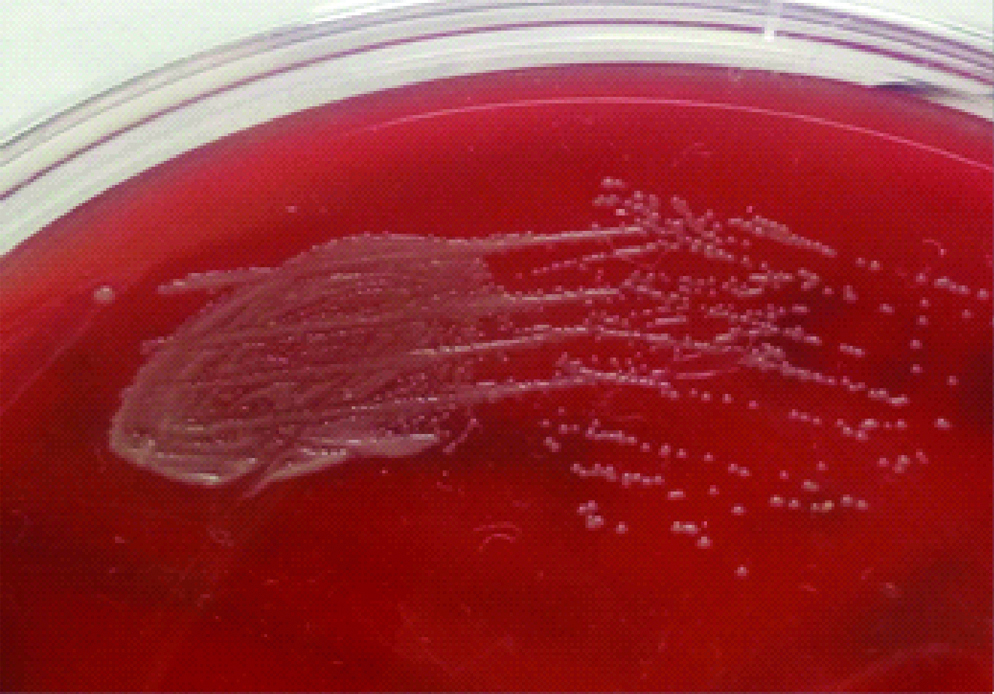
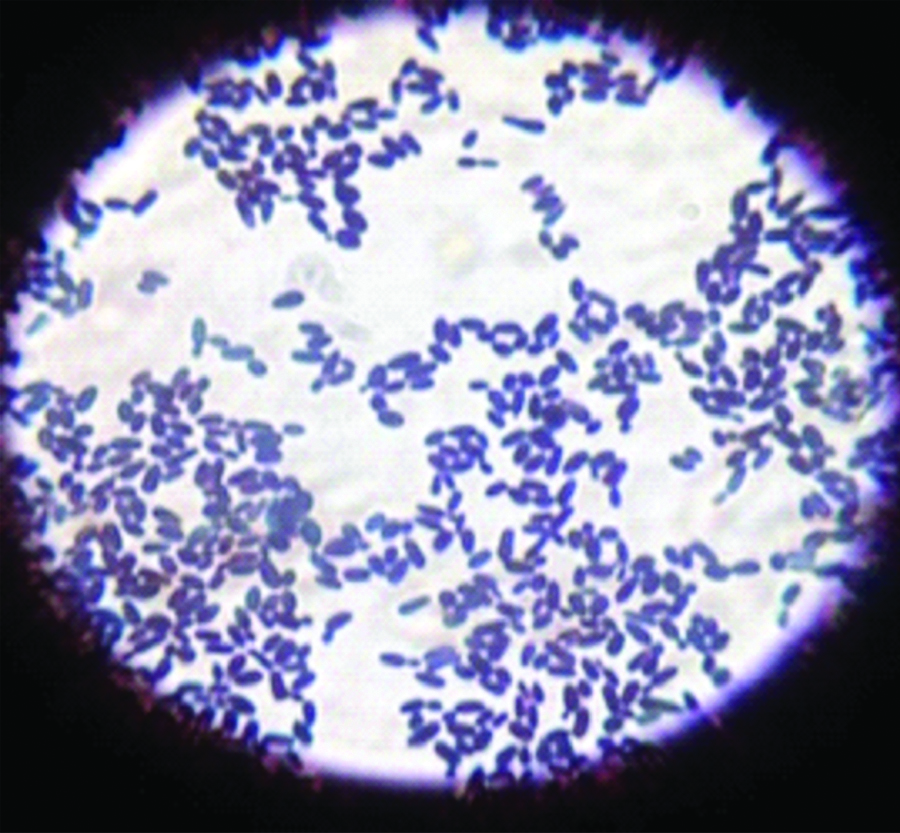
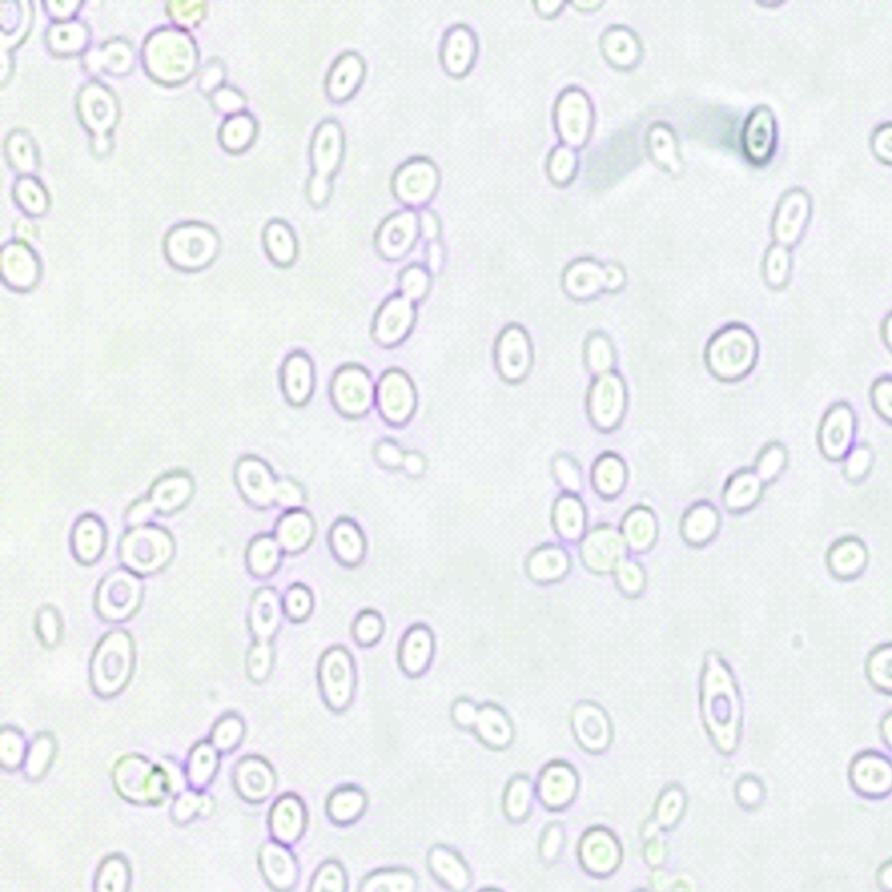
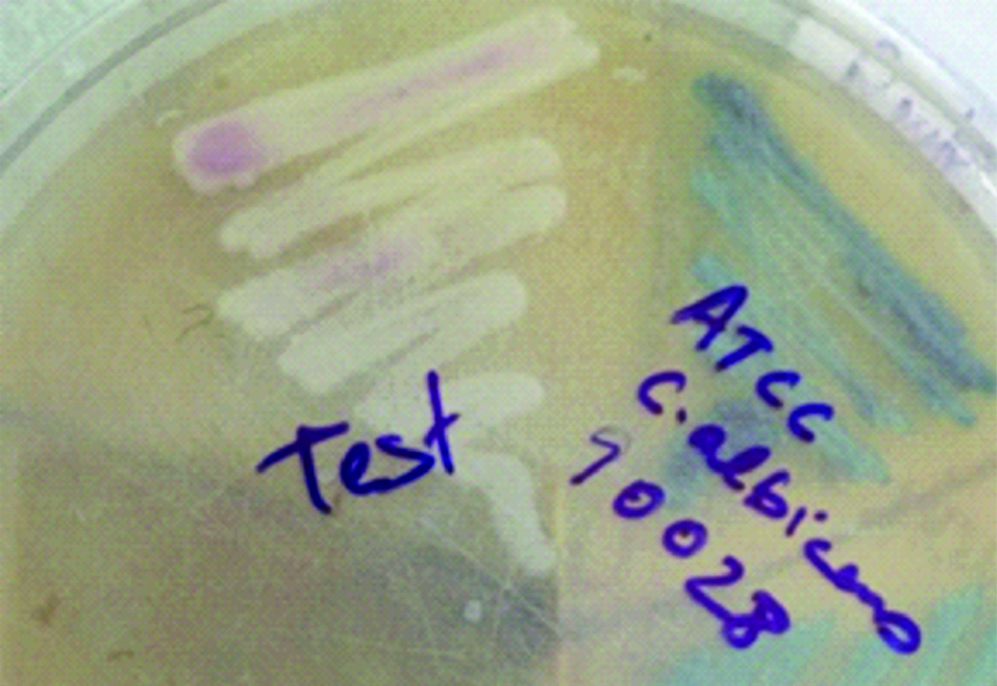
Blood was sent for culture and sensitivity. Blood culture was performed on BACTEC which signaled a positive growth within 24 hours of incubation. On subculture, tiny white opaque, non-haemolytic colonies were obtained on 5% Sheep Blood agar (SBA) [Table/Fig-1]. Gram stain revealed Gram positive budding yeast cells [Table/Fig-2]. Germ tube test was performed using Candida albicans ATCC 90028 as control. No germ tube was seen [Table/Fig-3]. The colony was sub-cultured on Hi Chrome agar (Hi Media), which revealed colourless colonies in 24 hours which became pink-purplish on further incubation [Table/Fig-4].
Tiny opaque colonies on SBA.
Gram stain showing Gram positive oval budding yeast cells (oil Immersion field).
Germ tube test showing no formation of germ tubes (40X).
Chrome agar (Hi Media) showing colony colour of isolate (test) after 48 hours of aerobic incubation.
Identification and sensitivity for colonies isolated was performed by Vitek 2 (BioMérieux Craponne, France). The yeast was identified as Candida haemulonii with 98% confidence. It was sensitive to Caspofungin (MIC<=0.25), Micafungin (MIC<=0.06), Voricanazole (MIC<=0.5) and intermediately sensitive to Flucytosine (MIC 8), while resistant to Fluconazole (MIC>=64) and Amphotericin B (MIC>=16). The case was presumptively diagnosed as Candida haemulonii blood stream infection. The patient was started on intravascular Caspofungin; 70 mg loading dose as slow infusion with normal saline and then 50 mg over an hour for 14 days. The yeast was further sent for confirmation of identification by Matrix Assisted Laser Desorption/Ionisation-Time Of Flight (MALDI-TOF). Score values were analysed as per manufacturer recommendations: a score of ≥2 indicates confidence to the species level, 1.7 to 1.99 indicates confidence to the genus level, and ≤1.7 indicated no identification. The yeast was identified as Candida auris with a score of ≥2. Antifungal susceptibility could not be confirmed by any other method. Considering antifungal susceptibility of Candida auris as per CDC guidelines (2017) [1], it was resistant to Fluconazole (MIC>32), Voriconazole (cross-resistance), Amphotericin B (MIC>2), but sensitive to Caspofungin and Micafungin (MIC<2 and MIC <4, respectively).
Patient Treatment and Follow-Up
After three days of antifungal administration (intravenous Caspofungin), patient showed remarkable response. Fever started subsiding with decreasing total leucocyte count. Subsequent blood cultures taken after completion of therapy were sterile and patient was clinically stable.
Discussion
This case report describes nosocomial blood stream infection caused by a rare fungus, Candida auris in a critically ill patient admitted in neurology ICU. The important risk factors for candidemia in this patient were intensive care settings, corticosteroid treatment and exposure to multiple antibiotics and mechanical ventilation. Other risk factors for Candida infections documented are AIDS, extensive burns, intravascular catheters corticosteroids and recent surgery, parenteral nutrition, use of advance life support on transplant patients [2]. Patients with Candida auris fungemia are more likely to have longer antecedent hospitalisation, underlying respiratory conditions, vascular surgery as compared to other Candida species in Indian ICUs and fungemia were also more frequently from public-sector ICUs of northern India [3].
Nosocomial candidiasis is taking the upper hand worldwide. However, a progressive shifting of trend from Candida albicans to non-albicans Candida has been witnessed in many countries because of rampant use of azole. Many of these resistant to traditional triazole sometimes cross-resistance to newer triazole, therefore, speciation and antifungal susceptibility testing of all yeast and fungi isolated from bloodstream or otherwise should be imperatively performed [2]. In this study, patient developed fever in spite of receiving multiple antibiotics. On obtaining a pure growth from BACTEC within 24 hours of incubation, simple tests like Germ tube test and Chromogenic agar helped in diagnosing the yeast as non-albicans Candida species. The colourless colonies on chromogenic agar appearing pink to purplish after 48 hours of incubation was not diagnostic of Candida haemulonii but hinted towards a rare pathogen. Candida auris also appears white, pink, or red in chromogenic agar [1]. For confirmation, VITEK 2 was employed for identification and sensitivity. Candida haemulonii has been reported to be identified by VITEK2 in earlier studies too [4]. Ruan SY et al., had described three C. haemulonii infections identified by VITEK 2 and confirmed by molecular analysis [5]. Oberoi JK et al., identified C. haemulonii in 97 blood cultures in two years by Vitek 2 [2]. However, misidentification of its related species as Candida haemulonii by Vitek2 has also been reported [Table/Fig-5] [6-9].
Details of misidentification of C.auris various studies [6-9].
| Year | Author | Country | Initial identification | Sample | Method used for confirmation | Confirmed identification |
|---|
| 2009 | Kim MN et al., [6] | Korea | Vitek2: C. haemulonii | 8-blood15-Chronic suppurative otitis media | Sequencing | Blood: C.haemulonii group I(1) C.psedohaemulonii (7)Ear: C.haemulonii sp nov |
| 2011 | Lee WG et al., [7] | South Korea | Vitek2: C. haemuloniiAPI 20C systems: Rhodotorula glutinis | Blood | Sequencing | C.auris |
| 2013 | Chowdhary A et al., [9] | India | Vitek2: C. haemulonii and C.famata | Blood | Sequencing | C.auris |
| 2014 | Khillan V et al., [8] | India | Vitek2: C. haemulonii | Pericardial fluid | Sequencing | C.auris |
| 2018 | Our report | India | Vitek2: C. haemulonii | Blood | MALDI-TOF | C.auris |
Intravenous Caspofungin was started presumptively for Candida haemulonii and the patient fortunately started improving clinically. However, MALDI-TOF results confirmed it to be a case of nosocomial fungemia by Candida auris. Considering, antifungal susceptibility of Candida auris as per CDC guidelines (2017) [1], it was resistant to Fluconazole (MIC>32), Voriconazole (cross-resistance), Amphotericin B (MIC>2), but sensitive to Caspofungin and Micafungin (MIC<2 and MIC <4, respectively). The results were similar to previous reports [6]. Contrastingly, CLSI-BMD (Broth microdilution method) gave inverse susceptibility results of that of Vitek 2 MIC in another study [7]. Similarly misleading high Amphotericin MIC has been reported earlier too [8]. Thus, multiple antifungal susceptibility method must be employed for such notorious pathogens.
Phenotypically, unlike C. haemulonni, C.auris grows well at 42°C but fails to grow in the presence of 0.01%-0.1% cycloheximide media [3,10]. It does not form pseudohyphae. Virulent factors like phospholipase and proteinase are produced by some strains. Clinically, C. auris fungemia is more virulent [10]. C. auris assimilates N-acetylglucosamine, succinate, and gluconate [11] and the risk factors although cover similar spectrum as discussed earlier, low Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) scores, duration of stay in hospital, duration of central line, total parental exposure and previous exposure to antifungals is more important in C.auris fungemia [3]. Notable difference is the susceptibility to Amphoterin B. C. auris is susceptible and C. haemulonii is resistant to Amphotericin B and azoles [8]. The overall mortality associated is higher in C.auris infections [3,10].
Emphasising the role of automation in this era, it has come a long way in not only decreasing the turn over time but also identification of rare and stubborn pathogens. However, limitations of every technique should be kept in mind and results should be interpreted collaboratively with other methods so as to avoid misidentification of hidden bugs like Candida auris. In this study, patient’s timely response to Caspofungin was undoubtedly positive. However, correct identification of Candida auris would have been missed without MALDI-TOF analysis.
All confirmed isolates of Candida auris should be reported to local and state public health officials and to CDC. Surveillance of laboratories serving healthcare facilities where cases of C. auris have been detected should also be conducted. Guidelines have also been formulated in cases of isolation of Candida auris from non-sterile sites and as coloniser [1].
C. auris causes widespread and persistent contamination of environmental surfaces within hospitals and is associated with both intra-hospital and inter-hospital transmission of clonal strains [6,9], therefore, infection control measures are vital to slowing the spread of Candida auris [10].
Conclusion
Candida auris behaves as an imposter and poses a threat for management of critical patients. A cautionary approach is advocated for reporting such rare and nosocomialy alarming bugs by automated instruments.
[1]. Candida auris interim recommendations for healthcare facilities and laboratories | Fungal diseases | CDC(2017) https://www.cdc.gov/fungal/candida-auris/recommendations.html [Google Scholar]
[2]. Oberoi JK, Wattal C, Goel N, Raveendran R, Datta S, Prasad K, Non-albicans Candida species in bloodstream infections in a tertiary care hospital at New DelhiIndian J Med Res 2012 136(6):997-1003. [Google Scholar]
[3]. Rudramurthy SM, Chakrabarti A, Paul RA, Sood P, Kaur H, Capoor MR, Candida auris candidaemia in Indian ICUs: analysis of risk factorsJ Antimicrob Chemother 2017 72:1794-801.10.1093/jac/dkx03428333181 [Google Scholar] [CrossRef] [PubMed]
[4]. Haider F, Begum R, Khare V, Yaqoob S, Shukla P, Das A, Candida haemulonii: an emerging pathogen in immunocompromised patient showing resistance to azoles and susceptibility to azolesEra’s J of Med Res 2016 3(1):45-46. [Google Scholar]
[5]. Ruan SY, Kuo YW, Huang CT, Hsiue HC, Hsueh PR, Infections due to Candida haemulonii: species identification, antifungal susceptibility and outcomesInt J Antimicrob Agents 2010 35:85-88.10.1016/j.ijantimicag.2009.08.00919786341 [Google Scholar] [CrossRef] [PubMed]
[6]. Kim MN, Shin JH, Sung H, Lee K, Kim EC, Ryoo N, Candida haemulonii and closely related species at 5 university hospitals in Korea: identification, antifungal susceptibility, and clinical featuresClin Infect. Dis 2009 48:e57-e61.10.1086/59710819193113 [Google Scholar] [CrossRef] [PubMed]
[7]. Lee WG, Shin JH, Uh J, Kang MG, Kim SH, Park KH, First three reported cases of nosocomial fungemia caused by Candida aurisJ Clin Microbio 2011 49(9):3139-42.10.1128/JCM.00319-1121715586 [Google Scholar] [CrossRef] [PubMed]
[8]. Khillan V, Rathore N, Kathuria S, Chowdhary A, A rare case of breakthrough fungal pericarditis due to fluconazole-resistant Candida auris in a patient with chronic liver diseaseJMM Case Rep 2014 1(3):1-5.10.1099/jmmcr.0.T00018 [Google Scholar] [CrossRef]
[9]. Chowdhary A, Sharma C, Duggal S, Agarwal K, Prakash P, Singh PK, New clonal strain of Candida auris, Delhi, IndiaEmerg Infect Diseases 2013 19(10):1670-73.10.3201/eid1910.13039324048006 [Google Scholar] [CrossRef] [PubMed]
[10]. Sears D, Schwartz BS, Candida auris: An emerging multidrug-resistant pathogenInter J Infect Diseases 2017 63:95-98.10.1016/j.ijid.2017.08.01728888662 [Google Scholar] [CrossRef] [PubMed]
[11]. Kathuria S, Singh PK, Sharma C, Prakash A, Masih A, Kumar A, Multidrug-Resistant Candida auris misidentified as Candida haemulonii: characterization by matrix-assisted laser desorption ionization–time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI Broth Microdilution, and Etest MethodEmerging Microbes and Infections 2015 53(6):1823-30.10.1128/JCM.00367-1525809970 [Google Scholar] [CrossRef] [PubMed]