Vertebral Cryptococcosis Infection in an Immunocompetent Host: A Rare Case Report
B Rajeshwari1, Salapathi Shanmugam2, Sadiya Niamath3, Mitra Ghosh4
1 Junior Consultant, Department of Histopathology, Apollo Speciality Hospitals, Vanagaram, Chennai, Tamil Nadu, India.
2 Junior Consultant, Department of Histopathology, Apollo Speciality Hospitals, Vanagaram, Chennai, Tamil Nadu, India.
3 Senior Consultant, Department of Histopathology, Apollo Speciality Hospitals, Vanagaram, Chennai, Tamil Nadu, India.
4 Senior Consultant, Department of Histopathology, Apollo Speciality Hospitals, Vanagaram, Chennai, Tamil Nadu, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. B Rajeshwari, Junior Consultant, Department of Histopathology, Apollo Speciality Hospitals, Vanagaram, Chennai-600095, Tamil Nadu, India.
E-mail: rajeshwari.buttannavar@gmail.com
Cryptococcosis is a systemic mycosis caused by yeast like fungus Cryptococcus neoformans. The infection results from inhalation of fungal spores in soil, house dust and in pigeon and other bird excreta. Infection usually occurs in immunocompromised individuals with lung and central nervous system being common sites of involvement. Skeletal involvement usually occurs as a component of disseminated cryptococcosis. Isolated skeletal involvement is rare. Here, the author presents a case of isolated skeletal cryptococcosis in an immunocompetent patient who presented with severe back pain and leg pain. Possibility of Tuberculosis of spine was suggested clinically but histopathology showed cryptococcal granulomas. Patient was HIV seronegative and did not have any other co-morbid conditions. There were no lesions in lung or brain. Because of increasing incidence of crytpococcal infections in immunocompetent hosts it should be included in the differential diagnosis of tuberculosis. Spine and fungal cultures should also be done. In the absence of fungal cultures, histopathological examination including the special stains is useful for diagnosis.
Disseminated cryptococcosis, Skeletal cryptococcosis, Tuberculosis spine
Case Report
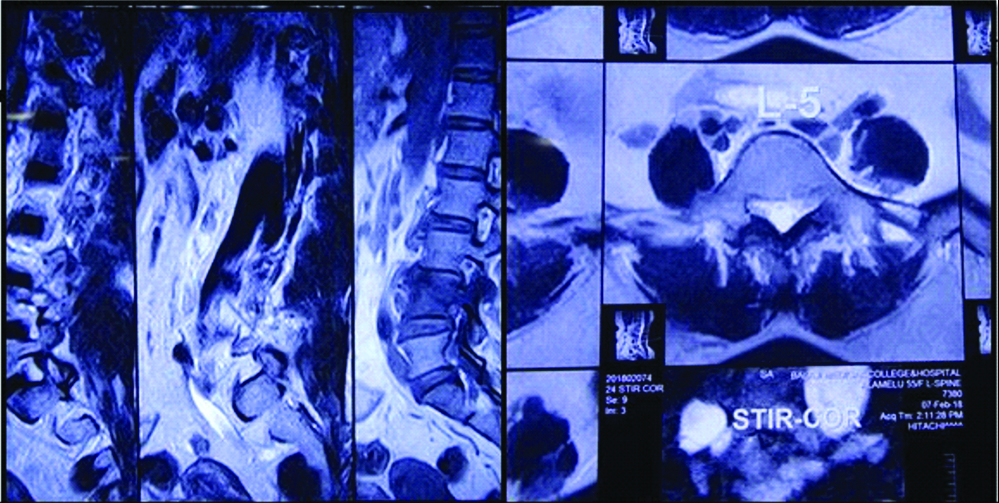
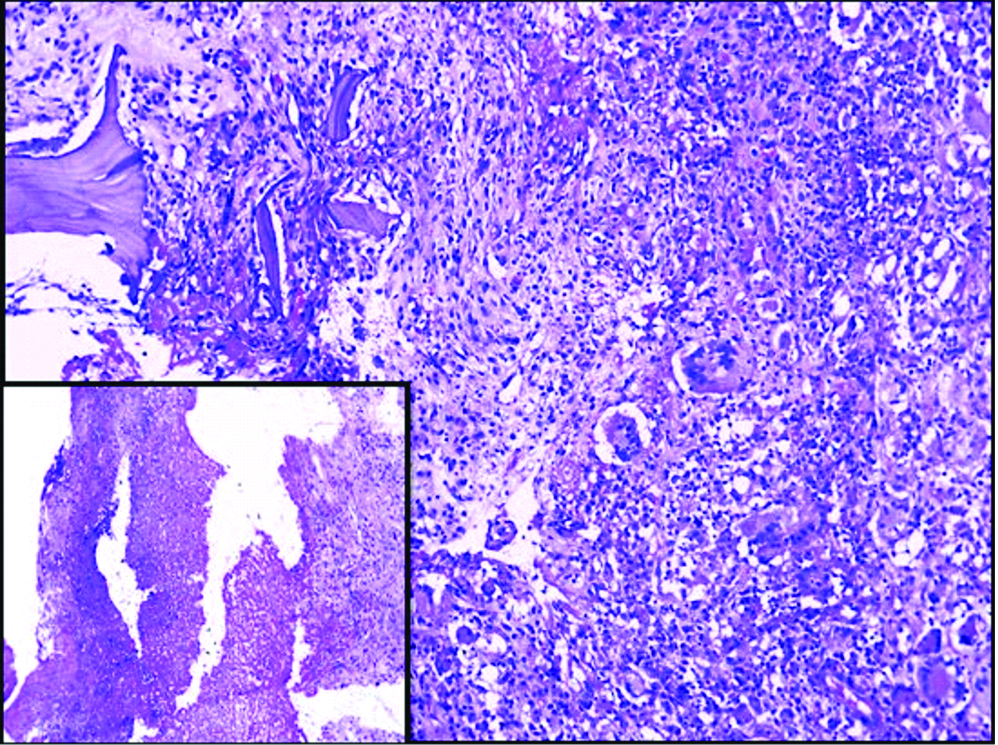
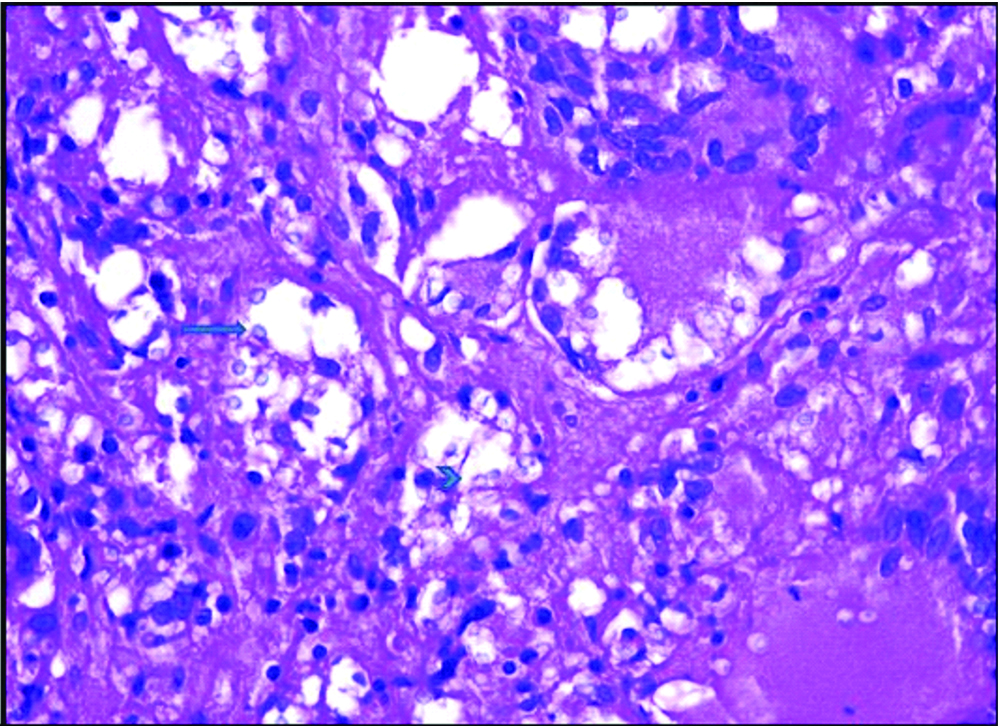
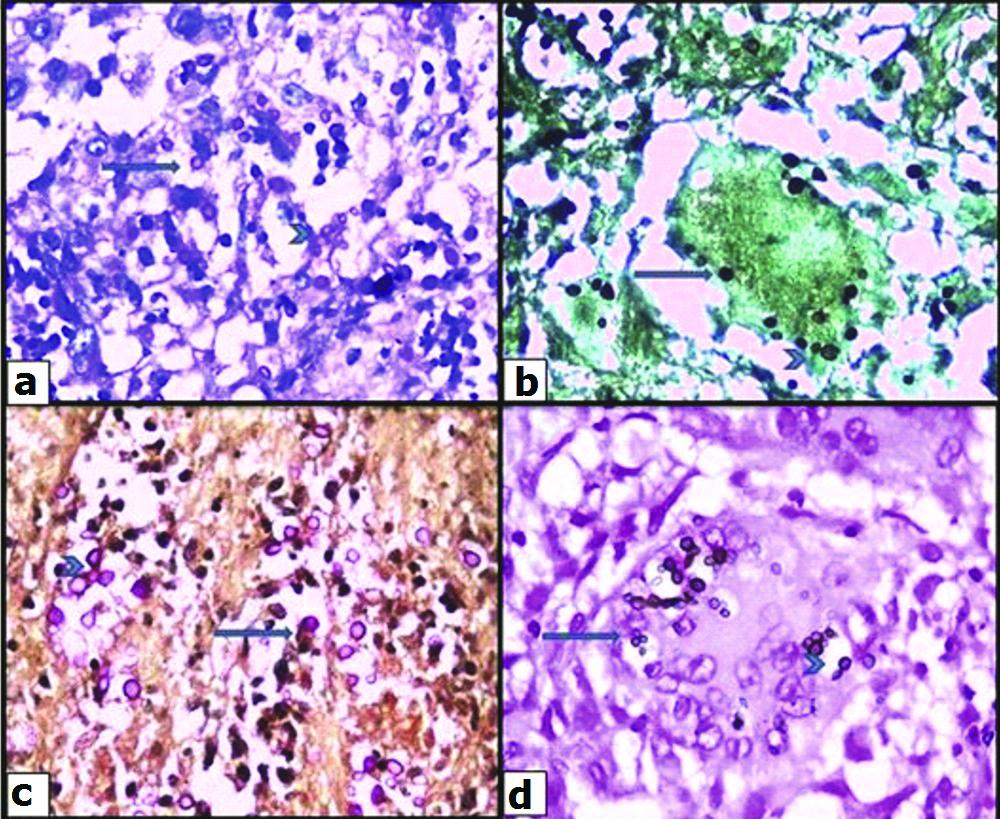
A 45-year-old lady, flower seller by occupation, presented with history of severe back pain and leg pain since 20 days which was not relieved by medication.There was no history of restriction of limb movements. There was no history of fever, cough, headache, loss of weight or loss of appetite, swelling or limp. The patient did not have any other co-morbid conditions. There was no significant past history. Clinical examination revealed no obvious swelling or deformity of spine. Knee and ankle jerk reflexes were normal. Patrick sign was negative. Systemic examination did not reveal any significant findings. MRI spine showed L4 spondylitis with edema involving the L4 vertebral body, transverse and spinous process. There was edema involving the psoas muscle at L4 level with a small collection in the distal end of the muscle [Table/Fig-1]. There was diffuse posterior disc bulge with facetal joint hypertrophy at L4/5 causing severe spinal canal stenosis and bilateral nerve root compression. MRI brain was normal. Chest X-ray did not reveal any lesion in the lung. Montoux test was negative. CSF analysis was not done as the patient did not have any symptoms of meningitis. The findings on MRI spine were suggestive of infective aetiology. Clinically, the possibility of tuberculosis was suggested initially. All the haematological parameters were within normal limits. Haemoglobin was 12.6 gm%, total WBC count was 9,400 cells/cumm with 62% polymorphs, 34% lymphocytes, 3% monocytes and 1% eosinophils. Absolute lymphocyte count was 3,200 cells/cumm. Platelet count was 2,57,000/cumm. ESR was 24 mm/1 hr. Patient was HIV seronegative. Blood culture was done and there was no growth observed on blood culture. No other tests or investigations for immunosuppression were done. Subsequently L4 laminectomy was performed and the tissue was sent for histopathological examination. There was no aspirate material available. Grossly the specimen showed multiple fragments of grey white soft tissue bits admixed with few bony spicules totally measuring 3×3×1.5 cm. Histological examination revealed fragments of bony trabeculae with areas of necrosis, epithelioid cells granulomas and multinucleated foreign body type of giant cells [Table/Fig-2]. There were varying sized yeast like fungal organisms with occasional narrow based budding and surrounded by clear space around them. These were seen predominantly extracellularly and focally within the histiocytes [Table/Fig-3]. Foci of neutrophilic exudate, and subacute inflammation were also noted. The fungal organisms were highlighted by fungal stains, Periodic Acid Schiff and Gomori methenamane silver (PAS and GMS). Mucicarmine and Masson’s Fontana stains were positive highlighting the capsule and melanin producing property of the fungus respectively [Table/Fig-4]. Stain for Acid Fast Bacilli (AFB) was negative. There was no tissue available for fungal culture. Cryptococcal antigen testing was not done. The case was reported was necrotizing granulomatous inflammation with cryptococcosis. Retrospectively patient was started on antifungal therapy with amphotericin B and fluconazole and she showed clinical improvement at the time of discharge but later the patient was lost for follow-up.
MRI spine showing edema involving L4 vertebra and psoas muscle with small collection at the distal end of muscle. Diffuse posterior disc bulge at L4/5 was seen.
Granulomatous inflammation with destruction of bone and necrosis inset H&E (100x).
Varying sized yeast like fungi intra and extra cellularly (arrow) with occasional budding (arrow head) H&E (400x).
Special stains to demonstrate crytpococci, PAS (a). GMS (b). Mucicarmine (c). Masson’s fontana (d) highlighting crytpococci (arrows) with occasional budding (arrows heads).
Discussion
Cryptococcosis is a systemic mycosis caused by Cryptococcus neoformans (C neoformans). It was also known as Torulosis, European blastomycosis or Busse-Buschke disease. This species was first isolated from peach juice by Sanfelice in 1894 [1]. It is a fungus commonly found in soil, house dust and in pigeon and other bird excreta [2]. Infection results from inhalation of spores, which germinate in pulmonary tissue and may disseminate via the bloodstream to the brain, meninges, bone marrow, and skin [3]. Less frequent is spread by direct inoculation through the skin [2]. Cryptococcus neoformans is budding yeast surrounded by a polysaccharide capsule that contains antigenic determinants permitting identification. It is distributed worldwide, existing in nature as a soil saprophyte [3]. It has two variant forms, Cryptococcus neoformans var. neoformans (CNVN) and Cryptococcus neoformans var. gatti (CNVG), both of which are pathogens in humans and animals [1]. Common presentations of cryptococcosis are related to pulmonary, Central Nervous System (CNS) and skin involvement. Rarely, liver, prostate and bone marrow involvement can occur [4]. Cryptococcus neoformans causes opportunistic infection in immunocompromised individuals [3]. The organism enters through inhalation route thus causing primary infection in lung or it can cause extrapulmonary infections via systemic spread. Bone involvement can occur in 10% of disseminated cases [5,6]. However, isolated osteomyelitis is very uncommon [5,7]. Infection in immunocompetent individuals is usually mild or subclinical; however overt infection has been described in 10-40% of cases involving non-HIV associated disease [8]. An increasing association of cryptococcosis in immunocompetent patients was observed in a few cases in India [9]. Cryptococcus infects an estimated 1 million people and results in approximately 625,000 deaths annually [10]. The overall incidence of cryptococcosis in immunocompetent individuals has been estimated at 0.2 per million per year [11].
Busse and Bushke, in 1894-1895, demonstrated for first time that Crytpococcus produced osteomyelitis of the tibia [11]. Patients typically present with swelling and pain of the surrounding soft tissue [8]. Skeletal involvement may occur as a component of disseminated cryptococcosis or secondary to underlying predisposing conditions such as sarcoidosis, tuberculosis, or other systemic disorders [12,13]. Vertebrae are the commonest bones involved though any bone can be involved [11]. In a review of 40 patients with skeletal infections caused by Crytpococcus, the vertebrae were the most common site involved, followed by femur, tibia and rib [14]. The vertebral involvement can mimic tuberculosis of the spine [11]. Other sites include humerus and skull [5]. Cryptococcal bone lesions, on radiology, show non specific findings consisting of osteolytic destruction of the vertebral bodies with paraspinal abscesses, thus simulating tuberculosis [11]. Disseminated crytpococcosis should be ruled out, before considering the diagnosis of primary cryptococcal osteomyelitis. The usual investigations done are chest X-ray, blood culture and Cerebrospinal Fluid (CSF) for culture, cryptococcal antigen testing and CSF India ink staining. Other investigations like urine cultures, sputum stain and culture and skin lesion cultures may also be done if applicable [15]. Diagnosis of bony infection requires biopsy. The organism appears as spherical, variable in size measuring 5-20 micrometers in diameter and appears as narrow-based budding yeast. The cells typically exhibit a thick polysaccharide capsule which is represented by an empty space surrounding the organism [8]. Several stains can be used to aid in the identification of the fungus including Wright’s stain, GMS, Calcoflour White, Alician blue, mucicarmine, Fontana Masson and India ink. Cryptococcal antigen positivity can also be useful in diagnosis [8]. Although testing for cryptococcal antigen in serum has high sensitivity and specificity, this test is of limited value because serum cryptococcal antibodies are present in only 30% to 40% of patients [14]. The level of antigen titre correlates with the severity of the disease [11]. Cultural and biochemical characteristics of the organism are essential for species identification [5]. Definitive diagnosis is established by culture of the fungus from infected material [8]. Some authors suggest that in the absence of positive blood culture demonstration of yeasts in biopsy material along with special stains like mucicarmine is sufficient to diagnose C.neoformans [8]. Immunodeficiency, be it primary deficiency or acquired, predisposes patients to infection by this fungus. A defective or insufficient interleukin-12 (a major cytokine in the initiation of T-cell mediated immunity) response, particularly IL-12 RB1 deficiency, has been reported in some cases [2]. Some authors suggested that cryptococcosis in immunocompetent populations is closely associated with genetic polymorphisms of some immune molecules (such as mannose-binding lectin, dectin-2, and Fcγ receptor IIB) which were associated with host immune functions like phagocytic activity and T cell responses which are required for fungal control and containment [13].
Isolated cryptococcal infection of bone is rare. Chen J et al., reported a case of crytpococcal infection of femoral bone which mimicked vascular tumour [5]. Sang J et al., reported a case of isolated iliac cryptococcosis in an immunocompetent host [13]. In both cases the patient presented with pain and sweeling with limp. Because of increased incidence of cryptococcosis in immunocompetent hosts, it should also be considered in the differential diagnosis of tuberculosis of spine. Histological examination and special stains are very useful for diagnosis of skeletal cryptococcosis in the absence of fungal cultures. It is rare to find isolated skeletal cryptococcosis in an immunocompetent host. One such case is presented here.
Conclusion
Crytptococcal infection of bone should be considered as a differential diagnosis along with tuberculosis since there is increased incidence of fungal infections in immunocompetent hosts. It is important to send tissue for fungal culture. Histology along with special stains are useful in the diagnosis in the absence of tissue for fungal cultures.
[1]. Zhou HX, Lu L, Chu T, Wang T, Cao D, Li F, Skeletal cryptococcosis from 1977 to 2013Front Microbiol 2014 5:74010.3389/fmicb.2014.00740 [Google Scholar] [CrossRef]
[2]. Firth GB, Ntanjana T, Law T, Cryptococcal osteomyelitis in a clinically immune-competent childSouthern African Journal of Infectious Diseases 2015 30(4):138-40.10.1080/23120053.2015.1107293 [Google Scholar] [CrossRef]
[3]. Minasian T, Hariri OR, Corsino C, Miulli DE, Farr S, Siddiqi J, Isolated lumbar-4 vertebral cryptococcosis in an immunocompetent patient-A case report and literature reviewCase Reports in Clinical Medicine 2013 2(6):348-50.10.4236/crcm.2013.26094 [Google Scholar] [CrossRef]
[4]. Taneja J, Bhargava A, Loomba P, Dogra V, Thakur A, Mishra B, Cryptococcal granulomas in an immunocompromised HIV-negative patientIndian J Pathol Microbiol 2008 51(4):553-55.10.4103/0377-4929.4376019008595 [Google Scholar] [CrossRef] [PubMed]
[5]. Chen J, Liu S, Xiong Z, Yang Y, Tan X, Luo Q, Cryptococcal infection of the femoral bone similar with pathologic features of vascular tumors: A case report and review of literatureInt J Clin Exp Pathol 2015 8(7):8551-54. [Google Scholar]
[6]. Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, Lancaster DJ, Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapyClin Infect Dis 2001 33(5):690-99.10.1086/32259711477526 [Google Scholar] [CrossRef] [PubMed]
[7]. Armonda RA, Fleckenstein JM, Brandvold B, Ondra SL, Cryptococcal skull infection: A case report with review of the literatureNeurosurgery 1993 32(6):1034-36.10.1227/00006123-199306000-000288327080 [Google Scholar] [CrossRef] [PubMed]
[8]. Mazumder SA, Achebe L, Heck R, Cleveland K, Cryptococcal ilium osteomyelitis and pelvic abscess in an immunocompetent hostJ Clin Case Rep 2018 1(1):1001 [Google Scholar]
[9]. Capoor MR, Nair D, Deb M, Gupta B, Aggarwal P, Clinical and mycological profile of cryptococcosis in a tertiary care hospitalIndian J Med Microbiol 2007 25(4):401-04.10.4103/0255-0857.3734918087095 [Google Scholar] [CrossRef] [PubMed]
[10]. Dash M, Padhi S, Sahu R, Turuk J, Pattanaik S, Misra P, Prevalence of cryptococcal meningitis among people living with human immunodeficiency virus/acquired immunodeficiency syndrome in a Tertiary Care Hospital, Southern Odisha, IndiaJ Nat Sci Biol Med 2014 5(2):324-28.10.4103/0976-9668.13617625097408 [Google Scholar] [CrossRef] [PubMed]
[11]. Houda B, Wafa A, Zoubida TM, Mohamed A, Mohamed A, Hicham H, Vertebral cryptococcosis in an immunocompetent patient-A case reportPan Afr Med J 2011 8:4210.4314/pamj.v8i1.7115822121450 [Google Scholar] [CrossRef] [PubMed]
[12]. Amenta PS, Stead J, Kricun ME, Case report 226: isolated Cryptococcus neoformans osteomyelitis of femurSkeletal Radiol 1983 9(4):263-65.10.1007/BF003541296867777 [Google Scholar] [CrossRef] [PubMed]
[13]. Sang J, Yang Y, Fan Y, Wang G, Yi J, Fang W, Isolated iliac cryptococcosis in an immunocompetent patientPlos Negl Trop Dis 2018 12(3):e000620610.1371/journal.pntd.000620629596420 [Google Scholar] [CrossRef] [PubMed]
[14]. Liu PY, Cryptococcal osteomyelitis: Case report and reviewDiagn Microbiol Infect Dis 1998 30(1):33-35.10.1016/S0732-8893(97)00190-9 [Google Scholar] [CrossRef]
[15]. Wood L, Miedzinski L, Skeletal cryptococcosis: Case report and review of the literatureCan J Infect Dis 1996 7(2):125-32.10.1155/1996/10210322514429 [Google Scholar] [CrossRef] [PubMed]