OLP is a chronic inflammatory disease that causes bilateral white striations, papules or plaques on the buccal mucosa, tongue and gingivae in which the immunopathogenesis involves cell-mediated immune dysregulation [1]. OLP affects between 0.5%-2.2% of the population and generally varies depending on geomorphology location [2]. Clinical manifestation can range from asymptomatic to a burning sensation or severe pain along with difficulty in phonation, mastication, and deglutition. OLP is categorised as a Potentially Malignant Disorder (PMD) of the oral mucosa (0-6.25%) [1,3]. Microscopically, it is characterised by hyperkeratosis, basal layer liquefaction of the epithelium with a dense infiltration of a band of lymphocytes [4,5].
Conjectured, causative factors like diabetes, stress, trauma and hypersensitivity to metals and drugs have different grade of support [6]. Contemporarily, viruses like HPV and human herpes virus have been associated in the pathogenesis of OLP. Previous research suggests apoptosis in oral epithelial cells of OLP [1] is analogous to a viral infection, where a virus acts as a cytoplasmic antigen resulting in a transfigured host cell protein profile [7]. Consequently, it must be heeded to investigate the role of HPV in the pathogenesis of OLP.
Inflated association between HPV16 and HPV18 with PMD and Oral Squamous Cell Carcinoma (OSCC) are reported [8-13]. HPV latency, reactivation or subclinical infection without discernible disease is not clearly understood [14]. HPVs cannot be cultured easily, all HPV detection assays rely strictly on the molecular analyses of HPV DNA. A variety of molecular methods are available for HPV DNA detection like in situ hybridization, southern blot, dot blot and PCR [15]. PCR is a beneficial aid used in research labs for diverse applications. PCR has been used for HPV detection, genotyping and viral load determination [16].
Till date, although a possible viral aetiology with HPV involvement with various oral lesions has been proposed by several authors, data reported in the literature concerning HPV infection in OLP remains highly controversial. No confirmatory association has yet been established between OLP and HPV. Therefore, it was decided to investigate the possibility of HPV presence in oral lichen planus and its possible aetiopathogenic role by utilising PCR coupled with HPV gene sequencing.
Materials and Methods
Collection of Tissue Samples
Around 30 biopsy samples comprising 15 oral lichen planus tissue biopsy and 15 normal buccal mucosa biopsies respectively, embedded in paraffin blocks were collected from different dental colleges located in Telangana erstwhile Andhra Pradesh. This study was carried out in the Department of Oral and Maxillofacial Pathology, MNR Dental College and Hospital and Sandor Life Sciences Pvt., Ltd., Hyderabad for a period of two years and two month from October 2012 to November 2014. Approval of the institutional ethical committee board was taken before pursuing the research project and written informed consent from the participants was obtained. A panel of certified Oral and Maxillofacial Pathologists reverified all the samples for histological diagnosis of oral lichen planus. A consensus diagnosis was reached in all the cases after examination of Haematoxylin and Eosin-stained sections. Sections of 5 micron thickness were cut from each wax block and only 3 microns were sectioned and stained with Haematoxylin and Eosin (H and E). The sections were stained by H and E as per the procedure laid down by Bancroft and Stevens [17].
DNA Extraction
The DNA was extracted from all 30 paraffin embedded samples. The tissue was removed from paraffin by dissolving in xylene and ethanol alternatively and vortexing. Supernatant of each sample containing residuals was air dried at room temperature before resuspending it in 150 μL tissue lysis buffer (ATL buffer from Qiagen). Proteinase K (50 μL of 40 mg/mL) was added to each samples, vortexed and incubated at 56°C in a water bath for 1-2 days until the samples completely lysed. After incubation, samples were centrifuged and vortexed in absolute alcohol and washed with 450 μL buffer (AW1 from Qiagen). Incubated at room temperature for 5-7 minutes and centrifuged at 18200 rpm for 3 minutes. Finally, the eluted DNA is passed through column during centrifugation and was collected in 1.5 mL tubes. The quality and quantity of DNA was evaluated by NanoDrop 2000 (Thermo Scientific) and stored at -20°C for downstream molecular applications.
Polymerase Chain Reaction (PCR)
For detection of L1 Gene of HPV, as described by Min KJ et al., the 145 bp DNA fragment was amplified by PCR and sense and antisense general primers [18]; 5’TTTGTTACTGTGGTAGATACCAC3’ and 5’GAATATGATTTGCAGTTTATTTTTC3’ respectively were added. The PCR mix containing 5x KAPA GC rich PCR buffer, 10 mM dNTPs, 10 pM each of sense and antisense primer, 5 U/μL KAPA2G Robust Taq DNA polymerase, 40 ng/μL DNA template and finally 5.8 μL of Milli-Q water (autoclaved) was added to make a final volume of 10 μL. The PCR reaction included the following steps: Pre Denaturation for 8 minutes at 96°C followed by 40 cycles of 45 seconds at 96°C, 50 seconds at 55.5°C and 50 seconds at 72°C and final extension for 11 minutes at 72°C for utilisation of extra dNTPs in mixture. The PCR product was visualised on 1.5% agarose gel.
Sequencing of PCR Product
The PCR products of 10 out of 13 patients were sequenced to know the subtypes of HPV. As described by Bell JR [19], PCR products were purified by using ExoSAP-IT. After purification step of PCR product, it was sequenced by Applied Biosystems 3130XL Automated Sequencer using the ABI Bigdye Ver 3.1. Sequence analysis comparison was performed using the Codon code Aligner 4.0.4 Software.
Statistical Analysis
The present study comprised 15 OLP and 15 controls cases with age ranging from 18 years to 56 years and comprising of eight males and seven female respectively. Subsequently, all subjects with OLP were pooled in two clinical groups: (1) Atrophic Erosive (AE) (atrophic, erosive, bullous, and mixed variants)-five Cases and (2) non atrophic-erosive (non-AE) (reticular, plaque-like, popular, and mixed non-AE variants)-10 cases. All the analysis was done using SPSS version 14.0 (Statistical Package for the Social Sciences). A p-value of <0.05 was considered statistically significant. Comparison of categorical variables was done using Chi-square test. The BLAST analysis result showed a 98% homology to HPV 18 of the positive specimen.
Results
Thirteen out of 15 (86.6%) lichen planus cases and none out of 15 controls were HPV 18 positive [Table/Fig-1]. Given the p-value gained by Chi-Square test (p<0.001) significant relation was observed between HPV-18 infection and oral lichen planus. Compared between males (p=0.002) and females (p=0.013) and controls significant relation was observed between sex and OLP too [Table/Fig-2]. However no significant relation (p=0.393) was observed based on the site of the lesion [Table/Fig-3] and based on type of lesion (p=0.082, p=0.3) [Table/Fig-4]. Statistical comparison with age was not computational.
Presence of HPV DNA among samples of OLP patients and healthy controls.
| Group | p-value |
|---|
| Cases | Controls |
|---|
| N | % | N | % |
|---|
| HPV | Not Present | 2 | 13.47% | 15 | 100% | <0.001; Sig |
| Present | 13 | 86.6% | 0 | 0% |
Relative Prevalence of HPV18 based on sex in OLP patients and healthy controls.
| Sex | Group | HPV | p-value |
|---|
| Not Present | Present |
|---|
| N | % | N | % |
|---|
| Female | Cases | 1 | 12.5% | 6 | 87.5% | 0.002; Sig |
| Controls | 7 | 100% | 0 | 0% |
| Male | Cases | 3 | 27.3% | 5 | 72.7% | 0.013; Sig |
| Controls | 8 | 100% | 0 | 0% |
Relative Prevalence of HPV DNA based on the site of lesion of OLP patients and healthy controls.
| Group | Site | HPV | p-value |
|---|
| Not Present | Present |
|---|
| N | % | N | % |
|---|
| Cases | Buccal mucosa | 1 | 25.0% | 9 | 72.7% | 0.393; NS |
| Labial mucosa | 2 | 50.0% | 4 | 27.3% |
| Tongue | 1 | 25.0% | 0 | 0% |
| Controls | Buccal mucosa | 15 | 100% | 0 | 0% | - |
| Labial mucosa | 0 | 0% | 0 | 0% |
| Tongue | 0 | 0% | 0 | 0% |
Relative Prevalence of HPV18 based on type of lesion of OLP patients.
| | Papular | Reticular | p-value |
|---|
| N | % | N | % | |
|---|
| Non-Atrophic | Not Present | 1 | 100% | 2 | 22.2% | 0.3; NS |
| Present | 0 | 0% | 8 | 77.8% |
| | Atrophic | Bullous | Erosive | p-value |
|---|
| N | % | N | % | N | % |
|---|
| Atrophic | Not Present | 0 | 0% | 1 | 100% | 0 | 0% | 0.082; NS |
| Present | 1 | 100% | 0 | 0% | 4 | 100% | |
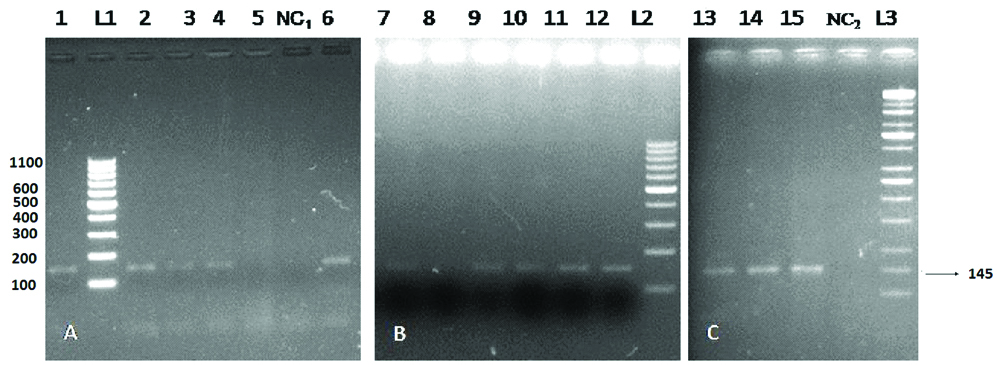
The DNA quantity and quality was determined by NanoDrop 2000 [Table/Fig-5]. First 15 readings indicate NanoDrop of the DNA from study group whereas 16 to 30 are for control group. The quality of DNA at 260/280 OD (Optical Density) was estimated ranging from 1.32 to 2.02 μg/mL, indicating good concentration of DNA. The PCR product was run on 1.5% agarose gel. No PCR product was amplified for negative samples (normal persons) as shown in [Table/Fig-6]. However, PCR product of 145 bp of HPV L1 gene was detected in 13 out of 15 patients [Table/Fig-7] indicating the presence of HPV in the Lichen Planus tissue of these patients. [Table/Fig-7] elucidates lane number 1 to 4, 6 and 7, 9 to 15; positive samples for HPV L1 gene (145 bp), lane number 5 and 8; negative for HPV L1 gene. Similarly lane number NC1 and NC2 are negative controls whereas, lane number L1 and L2 are 100 bp Ladder (Thermo scientific) and L3 is KAPA universal ladder. Out of 13 aforesaid samples first 10 samples were selected for sequencing to determine subtype of HPV. Sequence comparison of 10 HPV L1 genes by using BLAST revealed the sequences similar to HPV-18 L1 gene as referred on NCBI (Accession No. AY 262282). Comparison of sequences of HPV-18 L1 gene observed in present study with NCBI indicates 8 new SNPs in 145 bp PCR product [Table/Fig-8] which are highlighted by red colour. First, fifth and seventh SNPs were found common almost in all samples as compared to other SNPs, this may indicate a new variant of HPV-18 in Indian patients. The sequence of HPV-18 L1 gene in all 10 patients were compared with the sequences available (100) on NCBI site by BLAST alignment and found 97-99% sequence identity and not 100% [Table/Fig-9].
NanoDrop reading for all samples for determining the DNA quantity and quality.
| S. No | Conc. | 260/280 |
|---|
| 1 | 70.3 | 2.00 |
| 2 | 54.1 | 1.77 |
| 3 | 31.4 | 1.78 |
| 4 | 16.6 | 1.32 |
| 5 | 17.8 | 1.56 |
| 6 | 183.1 | 1.69 |
| 7 | 29.7 | 1.73 |
| 8 | 435.6 | 1.72 |
| 9 | 15 | 2.00 |
| 10 | 24.1 | 2.04 |
| 11 | 132.7 | 1.76 |
| 12 | 15 | 1.97 |
| 13 | 15.5 | 1.99 |
| 14 | 17.4 | 1.94 |
| 15 | 15.7 | 1.98 |
| 16 | 168.1 | 1.86 |
| 17 | 41.3 | 1.89 |
| 18 | 449.4 | 1.86 |
| 19 | 15.7 | 2.00 |
| 20 | 55.8 | 1.92 |
| 21 | 558.7 | 1.84 |
| 22 | 272.6 | 1.88 |
| 23 | 51 | 1.79 |
| 24 | 42 | 1.99 |
| 25 | 47.2 | 2.02 |
| 26 | 114.5 | 1.73 |
| 27 | 335.3 | 1.76 |
| 28 | 25 | 1.78 |
| 29 | 437.1 | 1.79 |
| 30 | 76.2 | 1.78 |
Agarose gel electrophoresis of HPV DNA exhibiting no band for HPV L1 gene in control patients, lane #1 to 15.
Lane # L indicates DNA 100 bp ladder.
: Agarose gel electrophoresis of HPV DNA: (A) and (B) gel picture lane #1 to 4,6,7,9 to 15 are positive for HPV L1 gene (145 bp).
Lane # 5 and 8 are negative for HPV L1 gene.
Lane # NC1 and NC2 are negative controls.
Lane # L1 and L2 are 100 bp ladder (Thermo scientific) and L3 is KAPA universal ladder.
Comparison of sequences of HPV-18 L1 gene observed in present study with NCBI indicates 8 new SNPs in 145 bp PCR product.
a) Lane # 1 indicates NCBI sequence,
b) Lane # 2 to 11 is as per sequence order
c) Nucleotide highlighted by red colour is SNPs detected in sequences.

Table showing sequence of HPV-18 L1 gene in all 10 patients comparing with the sequences available (100) on NCBI site by BLAST alignment.
| Patient ID | Total similar Sequences on NCBI | Sequence Identity |
|---|
| 1 | 100 | 97-99% |
| 2 | 100 | 95-97% |
| 3 | 100 | 97-99% |
| 4 | 100 | 97-99% |
| 6 | 100 | 97-99% |
| 7 | 100 | 97-98% |
| 9 | 100 | 97-99% |
| 10 | 100 | 97-99% |
| 11 | 100 | 97-98% |
| 12 | 100 | 97-99% |
Discussion
OLP is a chronic inflammatory disease of dysregulated cell-mediated immunity. OLP is categorised as a potentially malignant disorder (PMD) of the oral mucosa (0-6.25%) [3]. There is considerable variation in the geographical distribution of HPV in OLP in different parts of the world. HPV prevalence is higher in Asian and African [20] and less in northern and eastern side as demonstrated by Khovidhunkit SO et al., and Arirachakaran P et al, [21,22]. OLP can involve any site in the oral cavity, however, the main area involved are the buccal mucosa, gingiva and tongue [1]. The most common clinical presentation is whitish striae in a reticulated pattern. Reticular, papular, plaque-like, erosive, atrophic and bullous are six clinical types of OLP [10]. Classic histopathologic features include-lymphocytic infiltrate in the subepithelial region in band-like patterns, liquefactive degeneration of the basal layer, Civatte’s bodies (numerous eosinophilic colloid bodies), variable degrees of focal ortho or parakeratosis and irregular acanthosis [4,5].
HPV is a single circular double stranded DNA molecule and belongs to the family papillomaviridae and nearly 150 different HPV types are recognised [23]. HPV cause a wide range of infections, including common warts, genital warts, recurrent respiratory papillomatosis, low grade and high grade squamous intraepithelial lesions and cervical cancers. HPV 16 and 18 have increasingly been reported as being associated with potentially malignant disorders and oral squamous cell carcinoma. Infection by papillomaviruses occurs through micro wounds of the epithelium that causes virus to enter basal layer. The heparan sulfate mediates the first attachment of virions to cells. Since the basal layer consists of stem cells that are continuously dividing and this leads to the activation of a cascade of viral gene expression that results in 20 to 100 extrachromosomal copies of viral DNA per cell. This copy number is well maintained in undifferentiated basal cells all through the course of the infection. Owing to the above mentioned mechanism of infection there can be possible association between the HPV and OLP [24]. In leukoplakia HPV type 6, 11 and 16 are commonly associated and in OLP, HPV 16 and 18 are commonly associated. This indicates that as HPV being epitheliotropic they infect basal cells and can be involved in pathogenesis of the above mentioned lesions. Significant correlation has been established between presence of HPV and potentially malignant disorder such as oral leukoplakia [25].
A study by Flatharta O et al., showed 26.3% (9/38) prevalence of HPV-16 in OLP samples using PCR indicating a statistical association between HPV-16 and OLP indicating a possible aetiological role of HPV in OLP [26], but concluded stating that the results must be interpreted with caution, as from a biological sense, since this was not a universal finding in OLP and may be a chance occurrence.
Sand L et al., demonstrated 27.3% HPV DNA positivity in OLP using PCR of which five OLP were positive for HPV 18 and one OLP for nonspecific primers and they concluded that an association was demonstrated in the studied population but pathogenic influence of HPV infection cannot be elucidated [27].
Another study conducted by Campisi G et al., on HPV DNA in non atrophic/erosive OLP and atrophic/erosive OLP form showed a mean 19.7% positivity in exfoliated oral mucosa cells by nested PCR and the HPV genotype determined by direct DNA sequencing with predominance of HPV-18 [28]. They stated that the study confirmed higher risk of HPV infection in patients with OLP and owing to the oncogenic potential of HPV the presence of oral HPV infection should be determined in any variant of OLP.
Gonzalez JV et al., demonstrated that statistically significant relation of HPV in exfoliated cells of OLP in 50% of the sample (11/22) using PCR and also contributed to further evidence that oral infection with HPV can be a risk factor for preneoplastic and neoplastic oral lesions [25]. They also concluded that in addition to the viral role, other factors should be taken into account in the progression of HPV induced lesions, like immune compromise, genetic background and exposure to chemical or physical carcinogens. A long-term follow-up of potentially malignant lesions could help define the effective role of HPV in their aetiology owing to the fact that results from exfoliated cells may not demonstrate the HPV that mostly resides in basal cell layer of epithelium.
In another study, Mattila R et al., investigated 82 atrophic OLP for HPV DNA using Luminex-based assay and static cytometry [29]. HPV DNA was found in 15.9% of OLPs. There was a significant correlation between HPV DNA in OLP indicating an association with the subgroup of atrophic OLP. The significance of this observation remains obscure, because HPV 6 and HPV 11 are the two most common low risk HPV types which were present in samples of atrophic which progressed to carcinoma indicating malignant potential of low risk HPV which are generally implicated in benign lesions.
The present study showed HPV 18 positivity in 86.6% samples of OLP indicating a definite presence of HPV in oral lichen planus with non positive for HPV DNA in controls. Thirteen out of 15 lichen planus cases and none out of 15 controls were HPV 18 positive. Given the p-value gained by Chi-Square test (p<0.001), significant relation was observed between HPV-18 infection and oral lichen planus. Compared between males (p=0.002) and females (p=0.013) and controls significant relation was observed between sex and OLP too. However, no significant relation (p=0.393) was observed based on the site of the lesion. All erosive OLP showed HPV DNA positivity but variable positivity in non atrophic-erosive OLP due to the fact that atrophic-erosive types of OLP have more malignant potential than non atrophic-erosive OLP. Gonzalez JV et al., observed that only the erosive variant of OLP was positive for HPV [25]. However, no significant difference of HPV infection rate was evident when comparing the AE and non-AE OLP variants of OLP, a finding consistent with that of Sand L et al., and Campisi G et al., [27,28].
Likewise a study conducted by Wen S et al., to investigate the presence of HPV in northeast China in oral lesions like squamous cell carcinoma, candida leukoplakia, OLP using southern blot and PCR [30]. The study did not demonstrate any HPV in OLP indicating no role in pathogenesis of OLP in north eastern Chinese group of patient.
Two studies conducted by Khovidhunkit SO et al., and Arirachakaran P et al., on Thai patients using PCR to detect HPV 16 in OLP demonstrated only 1.54% (1/65) 2.7% (1/37) positivity respectively, indicating no association of HPV in OLP in Thai group of patients [21,22].
Razavi SM et al., also observed no significant difference between case and control groups in his study on HPV infection in OLP. 31% (9/29) HPV DNA and 7.1% (1/14) controls positivity was observed in OLP [31]. The results of the study correlated with few studies in the literature and showed a high positivity for HPV 18 (73.3%). Though previous studies have detected the presence of HPV in OLP; their results were not conclusive about their role.
A study conducted by Jalouli MM et al., found 46% prevalence in normal oral mucosa and study by Gichki AS et al., also reported HPV as 24 % in normal oral mucosa [32,33]; however in the present study none of normal oral mucosa samples were positive for HPV.
It can be theorize that keratinocytes expresses lichen planus antigen only at the lesion site. An early event in lichen planus lesion formation can be keratinocyte antigen expression induced by viral infection at the future lesion site. Upregulated Heat Shock Protein (HSP) expression by oral mucosal keratinocytes can be a common final pathway linking a variety of exogenous agents (systemic drugs, contact allergens, mechanical trauma, viral infection, bacterial products) in the pathogenesis of OLP [34].
In the present study HPV DNA was not detected in any of the normal control formalin fixed biopsies using PCR, which corresponds with the results of several studies [25,27,28,35]. Syrjanen in his review of HPV literature stated that pooled HPV detection rate in normal oral mucosa is 13% [4].
The present study findings suggest that high rates of HPV type 18 are observed in Indian patients compared to those of eastern and western continents. Our investigation detected the presence of HPV type 18 which could be associated with the pathogenesis of OLP and may also help in understanding the malignant potential of OLP. The current study also provided an insight into the critical differences between HPV infection and HPV presence as there was presence of HPV in the absence of any clinical findings that would correlate to it, thus indicating that OLP should be suspected with the presence of HPV in the absence of clinical correlation.
Limitation
The major limitation of the current study is the small sample size. The study should be conducted with the more number of cases to confirm the hypothesis of the relationship between HPV and OLP.
Conclusion
In the current study HPV type 18 was detected in 13 out of 15 cases of oral lichen planus which accounted for 86.6%. The study in addition to detecting the true presence of HPV in oral lichen planus by PCR coupled with HPV gene sequencing also provides an insight to understand the possible malignant potential of oral lichen planus.
The results obtained from the current study proved to be consistent in augmenting the already existing hypothesize and also imparting new concepts to evolve viruses, especially HPV for understanding the biological behaviour of oral lichen planus towards better management and treatment. To conclude, the results of our research affirm the fact that there is a definite presence of HPV in oral lichen planus adjudged by PCR coupled HPV gene sequencing.