Primary Squamous Cell Carcinoma (SCC) of the salivary glands is a very rare disease and establishing the true origin of an SCC manifesting in a salivary gland is always a daunting task, due to the natural proximity of salivary glands to surface epithelia. The aim of this report is to describe and discuss an SCC originating from the excretory duct of the submandibular gland with an unusual histological papillary appearance. A 39-year-old male presented with a slow growing mass in the floor of the mouth, which was clinically nodular, exophytic and ulcerated. Histological examination revealed a predominantly papillary architecture surrounding an intact salivary duct, which showed mucicarmine-positive material and no cell atypia. The duct was in a continuum with the neoplastic tissue, disposed as sheets of basaloid cells with foci of dysplastic squamous epithelium with minimal keratinization. The lesion was positive to CK14 throughout the parenchyma, CK7 in the luminal cells of ductal structures, CK13 in rare epidermoid areas and generally to AE1/AE3, highlighting foci of keratinization. The case reported herein is a very rare neoplasm in an unprecedented intraoral site, which emphasises the importance of histochemistry and immunohistochemistry in the diagnosis of rare lesions.
Case Report
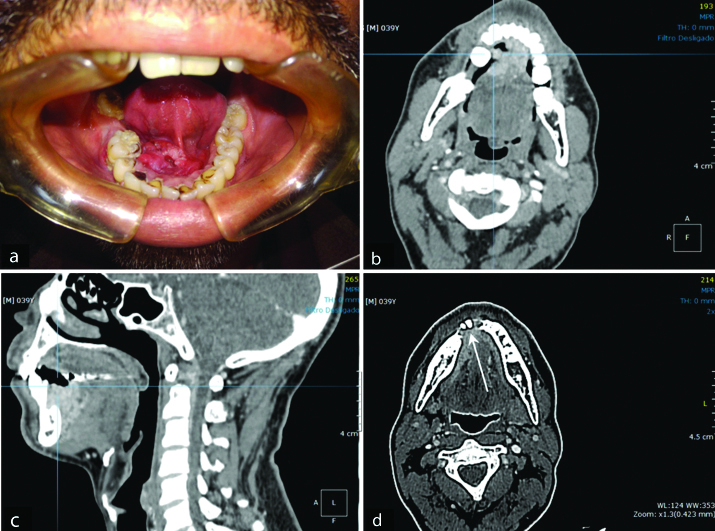
A 39-year-old Afro-Brazilian male presented with a one-year history of a slow growing mass in the anterior aspect of the floor of the mouth [Table/Fig-1a]. Clinical examination revealed a nodular exophytic and ulcerated lesion that was tender on palpation. On clinical examination, the lesion measured 20×30 mm, contained within the lining epithelium of the floor of the mouth, extending over the midline, involving the lingual frenulum and the base of the tongue. Despite the extent of the lesion, no cervical lymphadenopathy was detected on palpation, which was confirmed on CT imaging (T2N0M0). No other mucosal lesions suggestive of potential malignancy were detected intraorally nor was any paresthesia associated with the lesion.
Panel of clinical and CT images. a) clinical image of the lesion prior to the incisional biopsy showing considerable compromise of soft tissue in the floor of the mouth; b) (CT-transverse slices); and c) (CT-sagittal view) illustrate the extent of the tumour mass invading adjacent soft tissue structures (see skeletal muscles of the tongue irregularly adjacent to hyperdenser areas of neoplasm); d) (CT-transverse slice) shows bone invasion (arrow).
The patient was a rural worker with a 21-year history of smoking (20 cigarettes/day) and alcohol consumption on a regular basis, though the patient was vague to precise regularity.
The differential diagnoses established clinically were, from most likely to least likely, SCC, paracoccidiodomycosis, syphilis, tuberculosis or idiopathic ulcer.
A CT scan of the head and neck region showed a more extensive lesion than visualised clinically in the anterior portion of the floor of the mouth, which extended to the right side [Table/Fig-1b,c] with imaging features suggesting invasion of the mandibular bone [Table/Fig-1d]. From the CT images, the soft tissue lesion measured approximately 40×35×18 mm. No positive lymph nodes were detected on the CT images. The irregular hyperdense mass seen in the tongue muscles, together with cortical bone invasion anteriorly highlights the aggressive nature of the radiographic features, which increased the chances of malignancy in the differential diagnoses, thus, reducing the likelihood of infectious diseases. There was no evidence of compromise to vital structures as far as the images were concerned.
An incisional biopsy was sent for histopathological examination and the specimen was analysed using histochemical staining (H&E and mucicarmine) and immunohistochemistry for AE1/AE3, CK7, CK13, CK14 and Ki67.
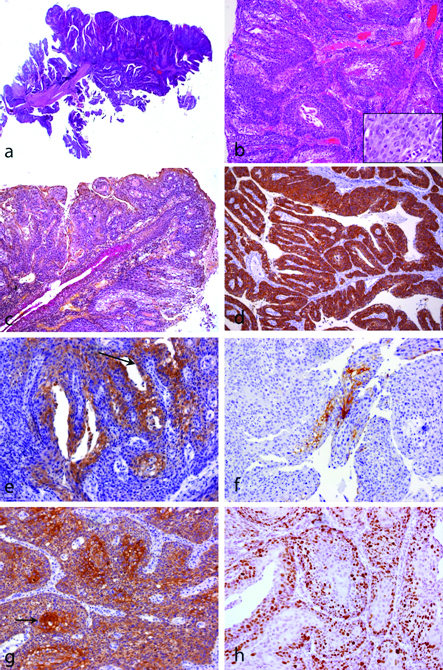
Histological examination revealed a tumour with a predominantly papillary architecture [Table/Fig-2a,b] surrounding an intact salivary duct with normal architecture, showing mucicarmine-positive material consistent with mucous [Table/Fig-2c] and no cell atypia. The duct was in a continuum with the neoplastic tissue, the latter disposed as sheets of basaloid cells with foci of dysplastic squamous epithelium with minimal keratinization [Table/Fig-2a,b]. Immunohistochemistry revealed a diffuse positivity to CK14 throughout the neoplastic parenchyma [Table/Fig-2d]; CK7 was evident in the luminal cells of ductal structures and in rare foci throughout the tumour mass [Table/Fig-2e]; positivity to CK13 was observed in rare epidermoid areas [Table/Fig-2f] and all parenchymal cells were AE1/AE3-positive highlighting foci of keratinization [Table/Fig-2(arrow)]. High nuclear positivity to Ki67 was observed indicating high proliferation of the tumour cells [Table/Fig-2h].
Squamous cell carcinoma of salivary gland duct: a) Low power view showing the papillary aspect of the tumour (H&E, X40); b) Islands of squamous cell carcinoma (X100). Inset: note atypical mitoses, nuclear pleomorphism and exuberant nucleoli (H&E, X400); c) Mucicarmine stain illustrates the secretion from the salivary gland duct, which is in a continuum with the carcinoma (X100); Immunohistochemistry reactions (d-h). d) CK14 is diffusely positive in the neoplastic cells (X100); e) CK7 is positive in the luminal cells (those lining the luminal spaces-arrow) and some areas of the tumour, which derived from the luminal cells, as seen in this photomicrograph (X200); f) CK13 is expressed by some squamous carcinoma cells (X200); g) AE1/AE3 staining illustrates the epithelial origin of the tumour and highlights foci of keratinization (arrow x100); and h) Ki67 was positive in numerous nuclei indicating high proliferation (X200).
The patient was referred to the local Head & Neck Surgery (H&N) service for evaluation. His tumour was staged at T2N0M0 at the time of referral. The patient was seen by the H&N team within three weeks of diagnosis, according to his hospital records, which also state that a preoperative evaluation was performed and new imaging requested. The patient was regrettably lost to follow-up since. Attempts to contact him proved fruitless and the regional hospital-based cancer registry was unable to identify a death entry that matched the identification data for this patient.
Discussion
Primary SCC is a very rare occurrence in salivary glands. In 2005, the World Health Organization (WHO) acknowledged that such neoplasm represents less than 1% of all salivary gland tumours, being 80% from the parotid gland and only 20% from the submandibular gland. The latest edition of the WHO Classification of Head and Neck Tumours (2017) [1], however, has established the parotid gland as the sole site for the extremely rare cases of SCC originating from the excretory duct.
Establishing the true origin of an SCC manifesting in a salivary gland is seldom an easy task due to the proximity of salivary glands to the surface epithelium, especially in the case of minor salivary glands, though it may also feature in major salivary glands, as demonstrated by Sobral AP V et al., [2].
Twenty-three cases of carcinoma arising from the Stensen’s duct have recently been reviewed by Nissanka-Jayasuriya EH et al., from which 10 were classified as SCC [3]. To that review, the authors added a case of their own, which presented architectural features of SCC, basaloid cells, very few foci of prickle cell differentiation and minimal keratinization in a continuum with a papillary pattern.
Similar in appearance though in a different site to the afore mentioned case, an SCC originating from the excretory duct of the submandibular gland with an unusual histological papillary appearance is herein reported. The lesion reported herein was diagnosed based on a combination of clinical, histological and immunohistochemical data, where continuity was detected between the neoplasm and the Wharton’s duct.
SCC arising from the submandibular gland is very rare and is estimated to comprise 1.8% to 3% of all submandibular malignant tumours [4,5]. No reports to date have demonstrated such an association between the neoplasm and the excretory duct of the submandibular gland [6,7].
The present lesion was located close to the orifice of the duct and showed papillary architecture histologically, intermingled with basaloid and squamous cells, which warranted further investigation for HPV. P16 was used as a surrogate marker of HPV infection, which proved negative. These are unusual features for those OSCC lesions arising from the surface epithelium, thus suggesting a different epithelial origin of the malignant cells. Histochemistry and immunohistochemistry were extremely useful to establish the final diagnosis. Histochemistry to mucicarmine illustrates the relationship between the neoplastic cells and the duct while a lack of mucicarmine staining is observed in the rest of the lesion, indicating no mucous production by the tumour. Furthermore, one might speculate that a lesion arising from the surface epithelium might reach the duct, however, in the present case the lesion is surrounding the duct without any evidence of invasion or destruction of the ductal cells themselves. This contributed to excluding a mucoepidermoid carcinoma, which would be the most important differential diagnosis in this case. Based on the papillary aspect of the tumour, Nissanka-Jayasuriya EH et al., proposed the possibility of malignant transformation of a ductal papilloma [3]. Indeed, the marked papillary features observed in such lesion resembled that of an intraductal papilloma.
The high expression of CK14 by nearly all parenchymal cells reinforced the diagnosis of SCC by excluding the possibility of a mucoepidermoid carcinoma, for which CK14-positive cells might be occasionally found lining the ductal structures of high-grade lesions only [2]. The neoplastic cells, on the other hand, seldom expressed CK7 [Table/Fig-2e]. Such feature is inconsistent with a diagnosis of high-grade mucoepidermoid carcinoma, as the latter usually expresses CK7-positivity in all cells (Sobral APV et al.,) [2]. Nonetheless, the fact that some cells were CK7-positive was strong evidence to attribute a glandular origin to the lesion. Although some OSCC in the oropharynx may be CK7-positive [8], which might suggest a transition point between types of epithelium (e.g., respiratory and squamous), squamous cell carcinoma from other sites within the oral cavity are not positive to CK7. Considering the likelihood of the tumour originating from stem cells close to the opening of the excretory duct [9], it is only natural that such neoplasms should be able to mimic the immunophenotype of both ductal structures and mucosal epithelium [2].
The present case report described a very rare neoplasm in an unprecedented intraoral site and emphasises the importance of ancillary methods, such as histochemistry and immunohistochemistry, in the diagnosis of rare lesions.
Compliance with Ethical Standards
All procedures performed in the patient reported herein were in accordance with the ethical standards of the institutional and/or national research committee (CNS resolution 466/12) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest: All authors declare no conflict of interest.
Informed consent: The patient gave written consent for data regarding his case to be used for teaching, education, research and publication purposes.
Funding
The authors would like to acknowledge FAPESP (São Paulo Research Foundation) and CNPq for financial support (FAPESP 2015/12418-5 and CNPq 304031/2014-3).
[1]. El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ, WHO Classification of Head and Neck Tumours. [Internet] 2017 4th edIARC:348El-Naggar AK, Chan JK, Grandis JR, Takata T, Slootweg PJ, editors [Google Scholar]
[2]. Sobral AP V, Loducca SVL, Kowalski LP, Santos IRB, Almeida OP, Araújo NS, Immunohistochemical distinction of high-grade mucoepidermoid carcinoma and epidermoid carcinoma of the parotid regionOral Oncol 2002 38(5):437-40.10.1016/S1368-8375(01)00089-6 [Google Scholar] [CrossRef]
[3]. Nissanka-Jayasuriya EH, Odell EW, Falconer DT, Stensen’s duct carcinoma with a papillary architectureHead Neck Pathol 2014 9(3):412-16.10.1007/s12105-014-0596-725480329 [Google Scholar] [CrossRef] [PubMed]
[4]. Kaname H, Yoshihara T, Yaku Y, Ishi T, Ultrastructure of primary squamous cell carcinoma of the submandibular glandMed Electron Microsc 1993 23(2):99-104.10.1007/BF02348034 [Google Scholar] [CrossRef]
[5]. Chen TC, Lo WC, Ko JY, Lou PJ, Yang TL, Wang CP, Rare involvement of submandibular gland by oral squamous cell carcinomaHead Neck [Internet] 2009 31(7):877-81.10.1002/hed.2103919360744 [Google Scholar] [CrossRef] [PubMed]
[6]. Elloumi-Jellouli A, Derbel F, Jellouli M, Ben Ammar S, Mrad K, Ben Romdhane, Primary epidermoid carcinoma of the submandibular salivary glandDermatol Online J 2005 11(1):26 [Google Scholar]
[7]. Gaikwad RV, Kumaraswamy SV, Keerthi R, Primary squamous cell carcinoma of the submandibular salivary gland: a rare entityJ Maxillofac Oral Surg [Internet] 2015 14(S1):57-59.Available from: http://link.springer.com/10.1007/s12663-011-0285-y10.1007/s12663-011-0285-y25861183 [Google Scholar] [CrossRef] [PubMed]
[8]. Shah AA, Jeffus SK, Stelow EB, Squamous cell carcinoma variants of the upper aerodigestive tract: a comprehensive review with a focus on genetic alterationsArch Pathol Lab Med [Internet] 2014 138(6):731-44.10.5858/arpa.2013-0070-RA24878013 [Google Scholar] [CrossRef] [PubMed]
[9]. Dardick I, Burford-Mason P, Current status of histogenetic and morphogenetic concepts of salivary gland tumourigenesisCrit Rev Oral Biol Med 1993 4(5):639-77.10.1177/10454411930040050201 [Google Scholar] [CrossRef]