Laparoscopic cholecystectomy has become the most popular surgery with respect to laparoscopic procedures over the past few years. It is the procedure of choice for patients with symptomatic gallstone disease. Several advantages have been documented such as increased patient satisfaction, shorter hospital stay and improved cosmesis. Although several studies report decreased postoperative pain as compared to open surgery, patients undergoing the procedure do experience considerable pain, especially in the first 24 hours postoperatively. This pain is treated using several analgesic regimens such as thoracic epidural analgesia, low-pressure pneumoperitoneum, non-steroidal anti-inflammatory drugs, opioids and intravenous paracetamol. Most commonly a multimodal analgesic regimen is recommended [1-4]. Transverse Abdominal Plane block (TAP block) is an analgesic technique used for abdominal surgeries. Rafi AN [5] in 2001 first described this technique and this proved to decrease the quantity of opioid consumed in the perioperative period especially for lower abdominal surgeries [6,7]. A variant of this block was the OSTAP block which provided effective pain relief for abdominal surgeries. Following this procedure, several side-effects of conventional analgesic techniques such as the side-effects of opioids, potential risk of haematoma, dural puncture and muscle weakness after epidural analgesia, inadequate pain relief was shown to be lowered. This block OSTAP is shown to be effective not just for laparoscopic cholecystectomies but also for other abdominal and renal procedures [8,9]. Not many studies have been published with OSTAP in laparoscopic cholecystectomies. However, the few that have been, conclude the beneficial effects on 24-hour opioid consumption. [6-9]. It has not yet been agreed upon as to what dose and volume of local anaesthetic infusion is needed to produce efficient analgesia in TAP block. In this study, OSTAP block was performed using ropivacaine 0.35% which has not been studied earlier. Since the transversus abdominis plane is not highly vascular, the volume of local anaesthetic used in this study was probably safe. OSTAP block using ultrasound guidance is becoming increasing popular for upper abdominal laparoscopic surgeries and hence authors planned to test the hypothesis that US-guided OSTAP blocks can reduce opioid consumption during the first 24 hours after laparoscopic cholecystectomy surgery.
The present study aimed to study the efficacy of OSTAP block with 0.35% ropivacaine in laparoscopic cholecystectomy when comparing it to the saline OSTAP block. The primary outcome measured was the assessment of pain by the Visual Analogue Score (VAS) in the first 24 hours postoperatively. Opioid requirement intraoperatively, in the PACU and in the first 24 hours, the time of discharge from PACU and incidence of postoperative nausea and vomiting were the secondary outcomes measured.
Materials and Methods
This was a prospective double blind randomised controlled trial conducted in tertiary hospital of Father Muller Medical College, Mangalore, Karnataka, India. The duration of study was one year from June 2017 to May 2018. The study included 42 American Society of Anaesthesiologists (ASA) physical status 1 and 2 patients, age ranging from 18-55 years, undergoing laparoscopic cholecystectomy under general anaesthesia. Institutional Human Ethics Committee approval (ECR/540/Inst/KA/2014/RR-17) and written informed consent was obtained.
Any patient who refused, who belonged to ASA physical status 3 and more, had history of allergy to local anaesthetics, had a platelet count<1,00,000 cells/cubic millimeter, an abnormal coagulation profile, infection at the site of needle insertion or sepsis were excluded from the study. Pre-anaesthetic checkup was carried out. Basic laboratory data was reviewed and once deemed fit for surgery, all patients were kept nil orally as per recent ASA guidelines [10]. Patients were randomly assigned to two groups of 21 each, Group A received a Subcostal transverse abdominal plane block bilaterally with a total volume of 40 mL of 0.35% ropivacaine, 20 mL on each side, following induction of general anaesthesia and before skin incision and Group B received the same block with 40 mL of saline [Table/Fig-1]. We used 20 mL of ropivacaine 0.35% bilaterally based on the study done by Breazu CM et al., who used 20 mL of 0.25% bupivacaine on each side and Bhatia N et al., who used 15 mL of 0.375% ropivacaine bilaterally [11,12].
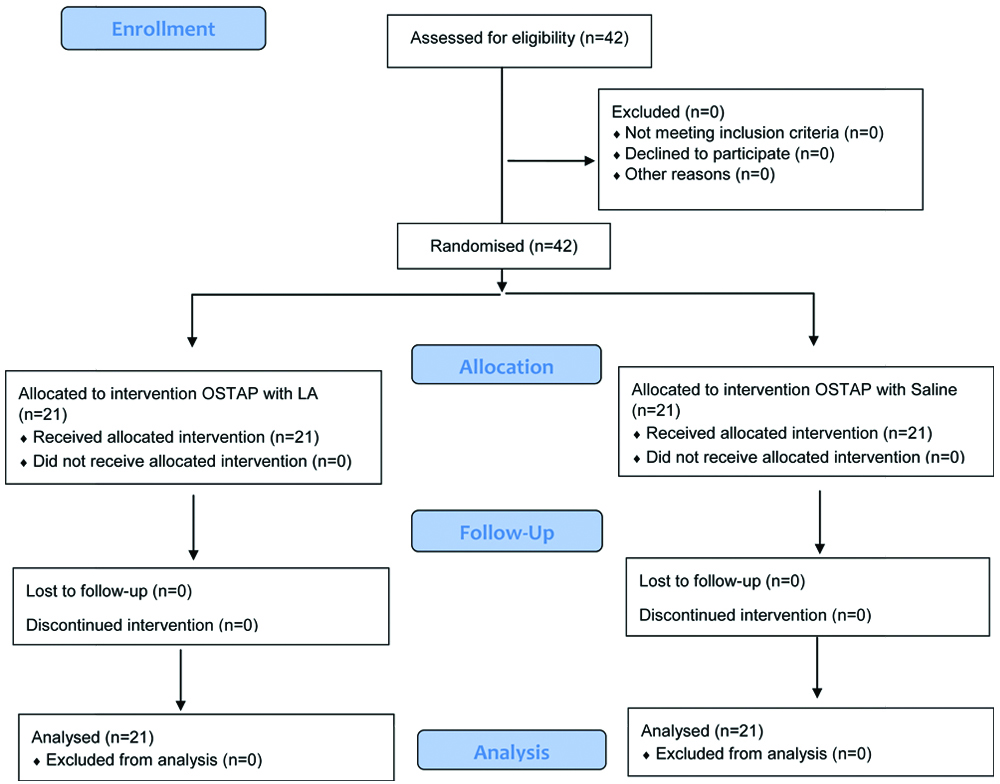
Sealed envelope technique was used for randomisation. The study design involved two anaesthesiologists. The first one randomised and allocated the patients to one of the groups. He/She was given 20 mL of either ropivacaine or saline (given to him by an experienced technician) which he was unaware of and loaded the drug/saline for the sub costal TAP block. The other performed the procedure, was blinded to the drug injected and monitored the intra/post op vitals, VAS [13], Modified Aldrete Score (MAS) [14] and PONV. On shifting the patients to the operation theatre monitors were connected. All patients were pre-oxygenated with 100% oxygen for a minimum of three minutes and pre-medicated with fentanyl 2 mcg/kg I.V. Propofol 2 mg/kg intravenously was used to induce anaesthesia and after confirming adequate mask ventilation, neuromuscular blockade was attained with 0.1 mg/kg of vecuronium I.V. Appropriate sized cuffed endotracheal tubes were used to secure the airway. Vecuronium in doses of 0.02 mg/kg i.v. was used for maintaining neuromuscular relaxation. Anaesthesia was maintained with air and oxygen and sevoflurane 1.5-2.0 dial concentration to achieve a Minimum Alveolar Concentration (MAC) of 1. Intraoperative monitoring of blood pressure, heart rate, oxygen saturation and electrocardiogram was done and recorded every five minutes.
The Sub-costal TAP block was performed under ultrasound guidance after administering general anaesthesia and prior to the skin incision. Patients received either local anaesthetic drug or saline. Following completion of the block, surgery commenced with introduction of the four supraumbilical ports. An increase in the Mean Arterial Pressure (MAP) and HR 15% above baseline values was treated with Fentanyl 50 μg I.V. as rescue analgesia.
Following surgery, reversal of residual neuromuscular blockade was achieved using neostigmine 0.05 mg/kg and glycopyrollate 0.1 mg/kg intravenously and trachea was extubated. Patient received paracetamol 1 gm IV at extubation and 8th hourly. Once the patient reached the postoperative ward oxygen saturation, blood pressure and heart rate was monitored. Monitoring of vital parameters such as pulse rate and blood pressure was continued for six hours postoperatively. Postoperative pain monitoring was done using the VAS and rescue analgesia in the form of Tramadol 1 mg/kg was given IV whenever VAS was > or =4. The two groups were compared with respect to the VAS, opioid consumption, Modified Aldrete score which was measured after 10 minutes, 30 minutes and one hour following extubation and incidence of post-operative nausea and vomiting. The total opioids consumed in the first 24 hours was recorded and compared.
Statistical Analysis
IBM Corporation-SPSS statistical package version 20 (SPSS) was used for statistical analysis, using the Independent t-test for the dependent variables, Chi-square test for parametric values, mean, standard deviation and T value. Data was expressed as mean±standard deviation, median and a p-value of <0.05 was considered significant. Assuming a 30% reduction in opioid use to provide 90% power at a significance level of 5% the sample size of 21 per group was calculated. Based upon prior studies which show 45-70% reductions in postoperative morphine requirement following TAP blockade, the 30% assumed reduction was a conservative estimate [15-18].
Results
Data from all the forty-two patients scheduled for surgery were included for analysis with none lost to follow-up. Demographic profiles of both groups were comparable as shown in [Table/Fig-2].
| Varibles | Group A | Group B | p-value# |
|---|
| (n=21) | (n=21) |
|---|
| Age (years)* | 45.45±14.12 | 40.05±11.91 | 0.192 |
| Sex (F/M)** | 11/10 | 16/5 | 0.107 |
| ASA I/II** | 14/7 | 18/3 | 0.147 |
| Weight (kg*) | 61.38±12.52 | 63.86±8.03 | 0.45 |
| Surgery time (minutes)* | 75.0±20.67 | 78.43±25.46 | 0.63 |
*Analysis done by independent students’ t-test;**analysis done using Chi-Square test; values are given as mean±standard deviation; #p-value>0.05=insignificant
There were statistically significant differences between the 2 groups in terms of the quality of postoperative analgesia. Group A (ropivacaine group) showed lower VAS score than the Group B (saline group) immediately after surgery (p<0.001), at 30 minutes, 2 hours, 4 hours, 6 hours and 24 hours [Table/Fig-3].
VAS pain scores with movement.
| Group A (n=21) | Group B (n=21) | p-value# |
|---|
| VAS 0 hour* | 0.71±1.05 | 2.21±1.61 | p=0.001 |
| VAS 30 minutes* | 1.3±1.62 | 2.30±1.35 | p=0.035 |
| VAS 2 hours* | 1.45±1.65 | 2.52±1.20 | p=0.025 |
| VAS 4 hours* | 0.90±1.44 | 2.29±1.55 | p=0.005 |
| VAS 6 hours* | 0.20±0.56 | 1.71±1.05 | p<0.001 |
| VAS 24 hours* | 0.38±0.60 | 1.52±0.98 | p<0.001 |
Independent students’ t-test used for analysis. VAS: Visual analog scale; *results are expressed as mean±SD; #p<0.05 was considered statistically significant
Intraoperative opioid consumption did not show significant difference in Group A as compared to Group B. Comparison of the intraoperative opioid fentanyl between the two groups’ shows that intra operative opioid fentanyl was higher in Group B with ‘a’ ‘t’ value of 0.566 but was statistically non-significant with a p-value of 0.575. Patients were given Inj. Tramadol i.v. whenever the VAS score was above 4 and the opioid consumption during the first eight hours after surgery (p<0.001) and total consumption within 24 hours show 5.89±6.13 versus 11.3±8.01 with p<0.05 with results expressed as morphine equivalent dose of tramadol [Table/Fig-4]. PACU stay was significantly less (p<0.001) in Group A with nearly all patients reaching discharge criteria within 30 minutes of reaching the PACU than in Group B who took longer [Table/Fig-5].
Comparison of the analgesic efficacy of OSTAP with ropivacaine 0.2% and OSTAP saline inlaparoscopic cholecystectomy.
| Group A (n=21) | Group B (n=21) | p-value# |
|---|
| Intraoperative opioid consumption of fentanyl (μg)* | 122±19.21 | 126.19±21.61 | p=0.575 |
| Opioid consumption in PACU first 8 hours*Morphine equivalent**(mg) | 4.64±3.38 | 9.52±3.76 | p<0.001 |
| Opioid consumption 8-16 hours*Morphine equivalent**(mg) | 1.25±2.75 | 0.83±2.14 | p=0.59 |
| Opioid consumption 16-24 hours*Morphine equivalent**(mg) | 0 (did not require) | 0.95±2.11 | p=0.042 |
| Total opioid consumption in 24 hours*Morphine equivalent**(mg) | 5.89±6.13 | 11.3±8.01 | p<0.05 |
Independent students’ t-test is used for analysis *results are expressed as mean±SD; #p-value<0.05 is considered statistically significant; **Monograph Tramadol. Palliative Care formulary. Available from www.palliativedrugs.com. Accessed on 12.04.2017
Modified Aldrete score in the post anaesthesia care unit.
| Varibles | Group A | Group B | p-value |
|---|
| 10 minutes | 7.76±0.7 | 7.43±0.67 | p=0.24 |
| 30 minutes | 9.29±0.784 | 7.59±0.95 | p<0.001 |
| 1 hour | 9.93±0.267 | 9.1±0.7 | p<0.001 |
Independent students’ t-test is used for analysis *results are expressed as mean±SD
Intraoperatively all patients received a standard 4 mg i.v. ondansetron and there was no significant difference in the incidence of postoperative nausea vomiting in the groups showing a p-value of 0.63 based on the antiemetic requirement [Table/Fig-6].
Antiemetic requirement between the two groups.
| Group A (n=21) | 5.9±2.72 | p=0.63 |
| Group B (n=21) | 5.52±2.36 |
Independent students’ t-test is used for analysis *results are expressed as mean±SD;
p-value>0.05=insignificant
Discussion
The causes of pain after a laparoscopic cholecystectomy are due to visceral and somatic components. Visceral pain is due to gallbladder dissection and the somatic pain is due to abdominal wall incisions and peritoneal cavity distention [19]. Managing the pain effectively is of paramount importance as it permits early mobilisation, reduces the postoperative complication rate and allows early discharge from the hospital. Effective postoperative analgesia is an important component of perioperative care. The conventional methods for analgesia after laparoscopic cholecystectomy consists of opioid administration, thoracic epidural analgesia and patient controlled analgesic techniques. Opioids are known to cause respiratory depression and late bowel movements [1] and epidural analgesia may have potential side-effects such as spinal hematomas, abscess or dural puncture [20]. Studies have shown that effective postoperative analgesic techniques improve surgical outcomes, reduce stress levels, provides better patient satisfaction with a reduction in opioid consumption and fewer side-effects [21,22]. The present study proves that oblique subcostal TAP block provides effective analgesia, decreases opioid consumption perioperatively and hastens recovery time following elective laparoscopic cholecystectomies.
Jianfeng MA et al., studied the analgesic efficacy of ultrasound- guided subcostal transverse abdominis plane block [23]. The study included 20 patients between 20 and 60 years of age undergoing elective laparoscopic cholecystectomy. They were given a left-sided subcostal TAP block with 0.25% levobupivacaine at a dose of 0.5 mL/kg, and came to the conclusion that, except for the lateral upper abdominal region, analgesia of prolonged duration was achieved at the anterior abdominal wall from the midline to axillary line. In 2016, a similar study was done by Breazu CM et al., where authors concluded that adequate analgesia up to 24 hours after laparoscopic cholecystectomy was provided to patients receiving the OSTAP block with bupivacaine 0.25% when combined with conventional multimodal analgesia regimen [11]. The present study further supports the evidence that subcostal TAP block provides superior analgesia for upper abdominal laparoscopic surgery. The present results are consistent with the mentioned studies and show the efficiency of OSTAP block using ropivacaine 0.35% for postoperative analgesia in laparoscopic cholecystectomy. The VAS scores were significantly reduced in Group A immediately after surgery and at 30 minutes, 2, 4, 6, 12 and 24 hours post-surgery (p<0.001). El-Dawlatly AA et al., compared the effect of conventional systemic analgesia against TAP block for laparoscopic cholecystectomy [24]. They also found a lower consumption of intraoperative opioids (8.6 μg vs 23 μg; p<0.01), and of morphine in the first 24 hours (10.5 mg vs 22.8 mg; p<0.05) in the TAP block group. Although, in the present study, intraoperative opioid consumption was higher in OSTAP saline group (126.19±21.61 μg) it was not statistically significant when compared to the OSTAP ropivacaine group (122±9.21 μg). However, the postoperative opioid consumption was significantly higher in the first 24 hours after surgery (morphine equivalent of tramadol in mg of 5.89±6.13 versus 11.3±8.01 with p<0.05). Ra YS et al., demonstrated that TAP with bupivacaine in laparoscopic cholecystectomy, reduced the numeric verbal pain score in the first 24 hours (p<0.001), the intraoperative and postoperative opioids consumption was also found to be lower (p<0.001) [19]. A study done by Tolchard S et al., compared the efficacy of subcostal transverse abdominis plane block with the conventional port-site infiltration in patients presenting for daycare laparoscopic cholecystectomies [25]. Tolchard S et al., found that TAP block effectively reduces opioid analgesic requirement in elective laparoscopic cholecystectomy and the burden of these patients on recovery services. It is noteworthy that these effects were achieved with basic ultrasound devices, unilateral blocks, and single injection subcostal TAP blocks. In the current study, OSTAP block with ropivacaine shortened the PACU stay time of most patients who received the subcostal TAP block achieving the MAS of nine within 30 minutes of termination of surgery. Authors consider that the amount of opioid used (intraoperative and postoperative as well) influences the PACU stay time. Patients needing less opioid reached an Aldrete score of nine, quicker than those who needed larger quantities. This suggested faster recovery time. Authors did not find any significant differences in PONV and also did not record any complications due to the OSTAP technique. A study was done in 2014 by Bhatia N et al., where one group received standard general anaesthesia and the other two groups received TAP block with 15 mL of 0.375% ropivacaine bilaterally either by posterior or subcostal approach [12]. Although initially, the subcostal and posterior TAP groups had comparable pain scores, after four hours these scores were significantly lower in patients who had received the subcostal TAP block.
The present study used similar drug doses and was found to have similar analgesic relief using OSTAP block.
Limitation
The study population was limited to one academic medical centre. Block onset time or its extension could not be assessed as the block was performed after induction of general anaesthesia. Future studies are required to evaluate the analgesic efficacy, recovery benefits and side-effect profiles, if any, of such blocks for all abdominal laparoscopic techniques. Furthermore, port site infiltration of local anaesthetic by the surgeon is not compared with the OSTAP and this would make an interesting area for further investigation. Recommended doses and volumes of local anaesthetics during the OSTAP block are not yet established. It would also be of interest to study different concentrations of local anaesthetic that are most effective for such axial blocks.
Conclusion
Authors conclude that Oblique Subcostal TAP with ropivacaine 0.35% can provide effective analgesia in the first 24 hours after laparoscopic cholecystectomy. The block has a significant opioid-sparing effect especially in the first 24 hours, enables faster recovery time and is relatively safe.
*Analysis done by independent students’ t-test;**analysis done using Chi-Square test; values are given as mean±standard deviation; #p-value>0.05=insignificant
Independent students’ t-test used for analysis. VAS: Visual analog scale; *results are expressed as mean±SD; #p<0.05 was considered statistically significant
Independent students’ t-test is used for analysis *results are expressed as mean±SD; #p-value<0.05 is considered statistically significant; **Monograph Tramadol. Palliative Care formulary. Available from www.palliativedrugs.com. Accessed on 12.04.2017
Independent students’ t-test is used for analysis *results are expressed as mean±SD
Independent students’ t-test is used for analysis *results are expressed as mean±SD;
p-value>0.05=insignificant