Introduction
The term IE is defined as a microbial infection of the heart valve (native or prosthetic) or the mural endocardium leading to tissue destruction and vegetation. Patients with cardiac abnormalities along with bacterial exposure (from trauma or other sources) leading to transient bacteraemia, are generally affected [1,2]. The diagnosis is made with the help of clinical manifestations, laboratory findings and imaging techniques (CT, MRI, Transoesophageal echocardiography). Clinical and laboratory findings include fever, elevated inflammatory markers, embolic phenomenon, heart murmurs, vascular and immunological phenomenon (splinter haemorrhages osler’s node, Roth spots, glomerulonephritis), elevated bilirubin, thrombocytopenia and lactataemia [1-4]. In the update on IE by Bashore TM et al., microbiological spectrum analysis showed that majority of the cases were caused by Staphylococci (coagulase negative staphylococci, S. aureus) and Streptococci (viridans streptococci). Minority cases were caused by Enterococci. The remaining few infections were caused by gram negative species, Candida species and MRSA [5], though the number is increasing recently due to prolonged hospitalisation, in-situ catheters and increase in lifestyle disease like diabetes. There were a sizeable number of culture negative cases as well [5]. The incidence of IE is found to be between 2 and 6 per 100,000 individuals per year, and that of associated mortality to be between 10% and 30%. Factors that affect mortality are type of pathogen, the underlying condition, and whether the infection is due to native or prosthetic heart valve [6-8].
The predisposing risk factors behind endocarditis are: history of rheumatic fever or rheumatic heart disease, prosthetic heart valve, congenital heart defect, previous cardiac surgery, history of IE, history of intravenous drug use and dental procedures [8,9]. In patients at a risk of IE, antibiotics should be taken before dental and certain surgical procedures such as lung surgery, surgery on infected skin, bone or muscle tissue and before any biopsy [9,10]. The new guidelines recommend prophylactic antibiotics in conditions that are associated with the highest probability of risk of IE (i.e., patients with prosthetic heart valve, congenital heart disease, previous IE and patients who develop cardiac valvulopathy after cardiac transplant) [6,8,11-13].
Antibiotics remain the mainstay for the treatment of IE. Quick initiation of antibiotic therapy is required to prevent valvular damage. Empirical antibiotic therapy is selected according to the most likely causative organism and based on standard recommendations. The treatment is sought for acute IE and certain cases of sub acute and culture negative IE. The main organisms targeted in native valve endocarditis and late prosthetic valve endocarditis are staphylococci, streptococci and enterococci and main organisms targeted in early prosthetic valve endocarditis and nosocomial IE are enterococci, methicillin resistant Staphylococcus aureus and gram negative pathogens [14,15]. Rapid and accurate diagnosis of the disease is essential in designing a therapeutic management strategy which otherwise may lead to prescribing inappropriate antibiotics and increasing the risk of antibiotic resistance. Antimicrobial resistance is evolving in the pathogens associated with IE and creates additional challenges for physicians [16]. The aim of this study was to analyse the agreement between the empirical therapy and the culture sensitivity reports.
Materials and Methods
This was an observational, prospective study extending from Nov 2013 to June 2014. The study was conducted in the cardiology department of a tertiary care hospital in Kochi, Kerala, India. The patients who were diagnosed to have either native or prosthetic valve endocarditis, based on the modified Dukes criteria, were included in the study [10,17,18]. All the 20 patients admitted in the cardiology department during the study period and who satisfied the study criteria were chosen in the study. Duke’s criteria for diagnosis of IE include:
Major criteria such as positive blood cultures consistent with IE, evidences that suggest endocardial involvement.
Minor criteria such as fever, predisposing factors, immunological phenomena, vascular phenomena, microbiological and echocardiographic findings that do not meet the specifications of major criteria but are consistent with IE.
A clinical diagnosis of IE requires two major criteria or one major and three minor criteria or five minor criteria.
Unconscious and disoriented patients, those on previous (in seven days preceding the inclusion) antibiotic therapy with symptomatic improvement and patients who did not fit into the modified Dukes criteria were excluded.
Information on demographic details, medical history, risk factors, specific pathogen, empirical antibiotic therapy, culture sensitivity report and definitive therapy were collected by the direct interview of the patient or bystander, assessing the patient medical profiles and by direct interaction with the physician. The presence of structural heart diseases, previous cardiac surgery, prosthetic valve, previous history of IE, other non cardiac issues (such as diabetes mellitus, asthma, inflammatory bowel diseases, chronic kidney disease, intravenous drug abuse, dental procedure), invasive procedures (such as endoscopy), type of valve involved, vegetation size, valve stenosis grade, presence of LV dysfunction are the relevant risk factors and medical history that we are looking at. Blood culture reports of the 20 patients were analysed to study the microbial spectrum. The collected data were transcribed into a special data collection form designed by the authors. The correlation between the empirical antibiotics and the risk factors of endocarditis were analysed from the collected data. The agreement between empirical therapy and definitive therapy was evaluated and documented. The data collected was tabulated and compared with other studies [15,18-24].
Approval of AIMS Institutional Ethics committee was taken before conducting the study (Thesis Review Committee/Pharma/2012/13). Only those patients who agreed to the informed consent were included in the study. Appropriate data collection form was prepared and collected by the direct interview of the patient or bystander, observation of patient medical profiles and by direct interaction with physicians. The collected data were compiled using Microsoft Excel and were presented in graphical format using piecharts, bar graphs etc. Statistical analysis has been carried out with Chi-square test.
Results
A total of 20 patients diagnosed with IE were included in the study. The demographic details of patients were analysed and mean age was found to be 48.7±9 years. Five (25%) patients were within the age of 41-50 years and four (20%) patients were within 51-60 years. Fourteen (70%) patients affected with IE were found to be males. There was a clear male preponderance. All patients were from the state of Kerala itself.
Analysis of clinical presentations showed that symptoms associated with IE were: fever in 15 (75%) patients, followed by dyspnea in two (10%) patients, and pedal oedema in one (5%) patient, heart failure in one (5%) patient and rheumatic fever in one (5%) patient.
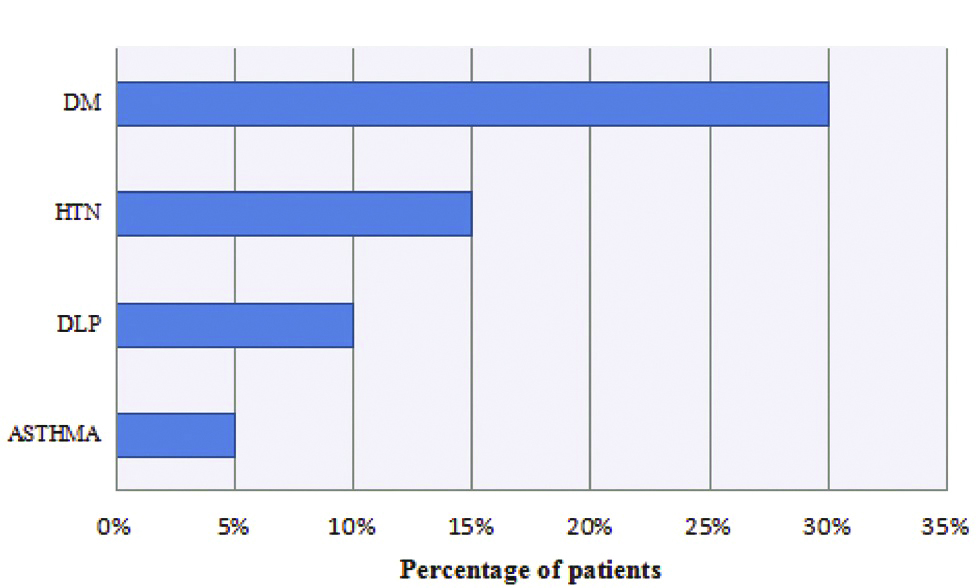
The presence of non-predisposing factors in the study sample was analysed. Following details were obtained [Table/Fig-1].
Patients with non predisposing conditions.
*DLP: Dyslipidemia; HTN: Hypertension; DM: Diabetes mellitus
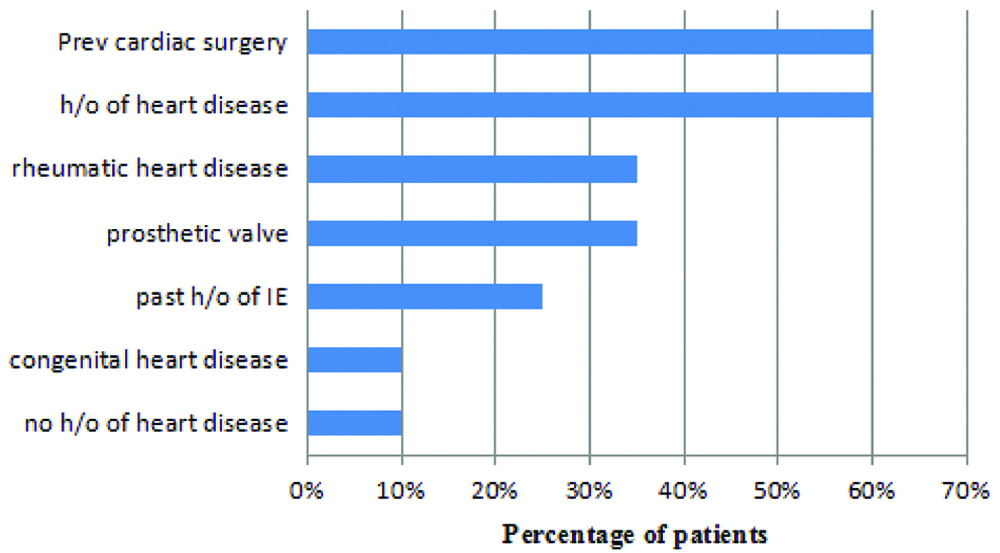
The presence of predisposing medical conditions in the study sample was also analysed. Following details were identified [Table/Fig-2].
Patients with predisposing medical conditions.
*H/o: History of; IE: Infective endocarditis
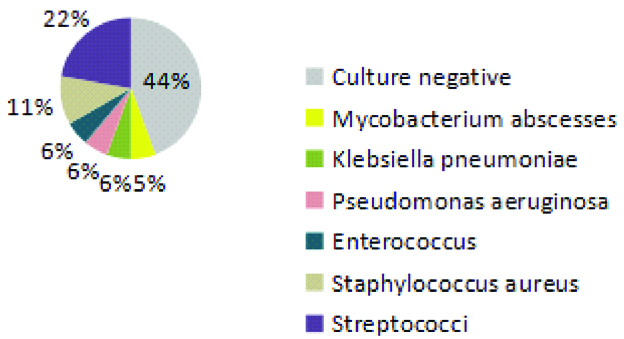
Blood culture reports of all patients were analysed. Streptococci were the most commonly isolated organism [Table/Fig-3].
Commonly isolated organisms from blood cultures of patients with IE.
Out of the total 20 patients, 13 (65%) were affected with native valve endocarditis and seven (35%) with prosthetic valve endocarditis. Among the patients with native valve endocarditis, 11 (84.6%) had mitral valve involvement and two (15.4%) had aortic valve involvement. All patients with prosthetic valve endocarditis had mitral valve involvement. Mitral valve was predominantly affected for 18 (90%) patients, while aortic valve involvement was found in seven (35%) patients. Out of the five (25%) patients who had vegetations, three (60%) had undergone previous cardiac surgery and the remaining had previous history of IE.
Empirical antibiotic regimens for IE were based on standard recommendations of European society of cardiology [22]. Reserved antibiotics were chosen according to the clinical situation and multiple predisposing factors, as shown in [Table/Fig-4].
Agreement between empirical and definitive therapy.
| Predisposing factors | Empirical therapy | Sensitive (S)/Resistant (R) | Native (N)/Prosthetic (P) | Organism isolated | Definitive therapy |
|---|
| RHD, Previous cardiac surgery, H/o heart disease | Vancomycin+Gentamycin | S | N | Mycobacterium abscessus | Vancomycin+Gentamycin |
| Previous cardiac surgery, H/o heart disease | Ceftriaxone | S | N | Streptococcus | Ceftriaxone |
| Past H/o IE, H/o heart disease | Deferred (blood culture awaited) | S | N | Streptococcus | Ceftriaxone |
| Past H/o IE, H/o heart disease | Piperacillin+Gentamycin | Meropenem-RGentamycin-R | N | Klebsiella pneumonia | Meropenem+colistin |
| Previous cardiac surgery | Cefepime+Ciprofloxaxin | Ciprofloxacin-R | N | Pseudomonas aeruginosa | Meropenem |
| RHD, Previous cardiac surgery | Deferred (blood culture awaited) | | N | Coagulase negative Staphylococcus | Crystalline pencillin+Gentamycin |
| Previous cardiac surgery | Crystalline pencillin+Gentamycin | S | N | Enterococcus | Crystalline pencillin+Gentamycin |
| No H/o heart disease | Ceftriaxone | S | N | Enterococcus | Vancomycin+doxycycline |
| Previous cardiac surgery | Gentamycin | | N | Culture negative | Ceftriaxone+gentamycin |
| RHD | Ampicillin+Gentamycin | | N | Culture negative | Ampicillin+Gentamycin |
| No H/o heart disease | Vancomycin | | N | Culture negative | Vancomycin, later linezolid |
| Prosthetic valves, Previous cardiac surgery | Cefotaxim+Ofloxaxin | | N | Culture negative | Cefotaxim+Ofloxaxin |
| No H/o heart disease | Deferred (blood culture awaited) | | N | Culture negative | Crystalline pencillin+Gentamycin |
| Prosthetic valves, Previous cardiac surgery | Linezolid | S | P | Staphylococcus | Levofloxaxin |
| Past H/o of IE, Prosthetic valves, Previous cardiac surgery | Ceftriaxone | R | P | Staphylococcus | Rifampicin+Vancomycin |
| RHD, Previous cardiac surgery, Prosthetic valves | Ampicillin+Gentamycin | S | P | Streptococcus | Ampicillin+Gentamycin |
| Prosthetic valves, Previous cardiac surgery | Amoxicillin+Clavulanic acid | Gentamycin-R | P | Streptococcus | Crystalline pencillin+Gentamycin |
| RHD, Previous cardiac surgery | Piperacillin tazobactum | | P | Culture negative | Piperacillin tazobactum |
| RHD, Prosthetic valves, Past H/o IE | Vancomycin+Amikacin | | P | Culture negative | Azithromycin+Ofloxacin |
| CHD, Previous cardiac surgery, Prosthetic valves | Deferred (blood culture awaited) | | P | Culture negative | Ceftriaxone |
H/o: History of; RHD: Rheumatic heart disease; CHD: Congestive heart disease; IE: Infective endocarditis
But in one case of prosthetic valve endocarditis, no antibiotic therapy was started empirically since the patient deferred the treatment.
From the microbiological data we found that gram positive cocci infections were the most predominant ones, in nine (45%) patients among 20 cases. Among the native valve and prosthetic valve infected patients, five (38.5%) patients and three (42.9%) patients respectively were found to be culture negative after obtaining the culture sensitivity report. Out of the native valve infected patients, four (30.7%) continued the therapy that was given empirically and three (23.1%) patients had their therapy deferred and in six (46.2%) cases, the antibiotic was changed according to the sensitivity report. Among those with prosthetic valve endocarditis, one (14.3%) patient had their therapy deferred and two (28.6%) patients continued empirical therapy after obtaining the sensitivity report and four (57.1%) cases had their antibiotic changed according to culture sensitivity report, as seen in [Table/Fig-4].
From this observation we found that reconsideration of empirical antibiotic selection is essential in the treatment of IE. Correlation of empirical and definitive therapy showed that in (15%) cases, therapy was continued without consideration of sensitivity pattern because of the patients’ adequate clinical response such as subsiding fever within one week of starting therapy and negative blood cultures within few days of starting antimicrobial treatment.
Complications of IE observed has been represented [Table/Fig-5].
Complications of infective endocarditis.
| Complication | No. of patients |
|---|
| Embolic phenomena | 6 (30%) |
| CVA/TIA | 3 (15%) |
| Renal failure (creatinine level elevated) After antibiotic | 5 (3-Gentamycin, 2-Vancomycin) (25%) |
| CHF | 2 (10%) |
Discussion
Very few studies have been conducted regarding the spectrum of IE in our country [17,25,26]. We analysed the demographic details, predisposing and non predisposing conditions, clinical features, microbiological characteristics, treatments and complications from which we tried to find the microbial spectrum of IE, predisposing factors and empirical antibiotic selections.
On analysing the demographic data we found that the mean age was found to be 48.7 years which is consistent with the results of a study by Kanafani ZA et al., where the mean age was 48 years [27]. There was a clear male preponderance (71%) among the study sample similar to the demographic reports of other studies [25,28,29]. This may be due to the cardioprotective effects of the female hormones on endothelial cell function and platelet aggregation thus decreasing the likelihood of developing IE in females [30].
The most common clinical presentation in the patients was fever consistent with the study by Garg N et al., [31]. The most common non predisposing medical condition was found to be diabetes mellitus followed by hypertension and dyslipidemia. This can be related to the study carried out by Duval X et al., in which 13% patients had diabetes mellitus out of the total study population [32].
Analysis of the predisposing factors revealed that rheumatic heart disease was the major element in our study group (35%) similar to the study by Khaled AA et al., (38%) [19]. There was no history of drug abuse and intestinal bowel disease in any of our patients. This suggests that IVDA (Intravenous Drug Abuse) is not yet a big social problem in the Indian community. Very interestingly, history of dental procedures was not present in any of our patients which were supported by other recent studies [20]. But this is in contrast to the study by Strom BL et al., which suggests that the main measure to prevent IE is by maintaining good oral hygiene [21]. Previous cardiac structural abnormalities were present in 75% of the population.
The diagnosis of IE was based upon the Modified duke’s criteria [17]. A positive culture of endocarditis and endocardial involvement was considered as the major criteria while predisposition, fever, microbiological evidence, vascular and immunological phenomena and echo findings were considered as minor criteria.
Out of the total population, 65% were diagnosed with native valve endocarditis which is in close agreement to the study by Tugcu A et al., in which 97% of their patients had native valve endocarditis [26]. A substantial portion of patients had prosthetic valve endocarditis. This involvement of prosthetic valves was consistent with the large body of data from established series in published literature [10]. Oliver R et al., has observed that the risk of developing IE is five times more in patients with prosthetic valves compared with native valves [22].
We should understand that many of these patients might have already been prescribed inappropriate antibiotics due to improper diagnostic work up of the illness. This is an issue not only in the developing world but has also been reported in the developed world [33]. The most commonly identified organisms in cultures were the Streptococcus and Staphylococcus species. This change in trend may be due to changing patient characteristics [34,35].
Gram positive cocci infections were found to be more predominant. Newer trends in microbiological spectrum like Mycobacterium abscessus, Klebsiella pneumonia, and Pseudomonas aeruginosa were also found.
Culture negative cases were seen in 40% of the population and this most likely represents prior antibiotic therapy. The rate of culture negative reports range from 2.5 to 31% of all cases [18,23] seen in various studies. According to Habib G et al., empirical treatment should be started promptly on suspicion of infection, and blood cultures should be drawn before the initiation of antibiotics [15]. Selection of antibiotics should be based upon whether there was a previous exposure to antibiotic therapy, whether the native or prosthetic valve is affected and on the local epidemiological data on antibiotic resistance [24].
Empirical therapy for IE was based on standard recommendations and higher antibiotics chosen according to the clinical situation. Majority of the patients with native as well as prosthetic endocarditis had undergone a change in their therapy according to the culture sensitivity report which has become a major challenge to the physicians for further treatment.
When compared with other studies, the complications of IE were less in our study [7,36-38]. The incidence of stroke was quite low compared to the western reports [39,40].
Important changes have been observed in the demographic, clinical and microbiological profile of IE patients throughout this study. These changes point to the fact that the disease now needs a new step, particularly related to treatment options regarding empirical antibiotic selections.
Limitation
The study was a single centre study. There were sample size limitations as the disease is of low incidence. Since serological and molecular techniques are not in common use in the developing world, our results are not showing the ‘true’ microbiological profile of the disease. There is inherent selection bias as the data is obtained from a tertiary care setting. The data cannot be generalised to the community. No follow-up data were available after discharge of patients from the hospital.
Conclusion
In our study, previous cardiac surgery, prosthetic valve and rheumatic heart disease were found to be the major predisposing factors. The most commonly identified pathogens in cultures were Streptococcus species and Staphylococcus species. Culture negative cases of IE were seen in 40% of the patients which most likely indicates prior antibiotic therapy or lack of sophisticated diagnostic techniques. Altering antibiotics, after culture reports, may lead to increase in unintended antibiotic resistance development. So, empiric antibiotic regimen should be reconsidered based on local epidemiology. Isolation of atypical pathogen indicates a changing microbiological spectrum of this disease.
H/o: History of; RHD: Rheumatic heart disease; CHD: Congestive heart disease; IE: Infective endocarditis
[1]. Thuny F, Disalvo G, Belliard O, Avierinos JF, Pergola V, Rosenberg V, Risk of embolism and death in infective endocarditis: Prognostic value of echocardiography: A prospective multicenter studyCirculation 2005 112(1):69-75.10.1161/CIRCULATIONAHA.104.49315515983252 [Google Scholar] [CrossRef] [PubMed]
[2]. de Isla LP, Zamorano J, Lennie V, Vázquez J, Ribera JM, Macaya C, Negative blood culture infective endocarditis in the elderly: Long-term follow-upGerontology 2007 53(5):245-49.10.1159/00010169117429212 [Google Scholar] [CrossRef] [PubMed]
[3]. Yu CW, Juan LI, Hsu SC, Chen CK, Wu CW, Lee CC, Wu JY, Role of procalcitonin in the diagnosis of infective endocarditis: A meta-analysisAm J Emerg Med 2013 31:935-41.10.1016/j.ajem.2013.03.00823601504 [Google Scholar] [CrossRef] [PubMed]
[4]. Bruun NE, Habib G, Thuny F, Sogaard P, Cardiac imaging in infectious endocarditisEur Heart J 2013 35(10):624-32.10.1093/eurheartj/eht27423900698 [Google Scholar] [CrossRef] [PubMed]
[5]. Bashore TM, Cabell C, Fowler Jr V, Update on infective endocarditisCurrent Problems in Cardiology 2006 31(4):274-352.10.1016/j.cpcardiol.2005.12.00116546554 [Google Scholar] [CrossRef] [PubMed]
[6]. Allen U, Infective endocarditis: Updated guidelinesPaediatrics & Child Health 2010 15(4):205-08.10.1093/pch/15.4.20521455464 [Google Scholar] [CrossRef] [PubMed]
[7]. Aggarwal M, Vijan V, Vupputuri A, Nandakumar S, Mathew N, A rare case of fatal Endocarditis and sepsis caused by Pseudomonas aeruginosa in a patient with chronic renal failureJCDR 2016 10(7):OD1210.7860/JCDR/2016/20220.817527630891 [Google Scholar] [CrossRef] [PubMed]
[8]. Baddour LM, Wilson WR, Infections of prosthetic valves and other cardiovascular devicesMandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases 2005 Philadelphia, PaElsevier Churchill Livingstone:1022-44. [Google Scholar]
[9]. Ellis ME, Al-Abdely H, Sandridge A, Greer W, Ventura W, Fungal endocarditis: evidence in the world literature, 1965-1995Clin Infect Dis 2001 32(1):50-62.10.1086/31755011118386 [Google Scholar] [CrossRef] [PubMed]
[10]. Li JS, Sexton DJ, Mick N, Nettles R, Fowler Jr VG, Ryan T, Proposed modifications to the Duke criteria for the diagnosis of infective endocarditisClin Infect Dis 2000 30(4):633-38.10.1086/31375310770721 [Google Scholar] [CrossRef] [PubMed]
[11]. Piper C, Körfer R, Horstkotte D, Prosthetic valve endocarditisHeart 2001 85(5):590-93.10.1136/heart.85.5.59011303018 [Google Scholar] [CrossRef] [PubMed]
[12]. Sexton DJ, Tenenbaum MJ, Wilson WR, Steckelberg JM, Tice AD, Gilbert D, Ceftriaxone once daily for four weeks compared with ceftriaxone plus gentamycin once daily for two weeks for treatment of endocarditis due to penicillin-susceptible streptococciClin Infect Dis 1998 27(6):1470-74.10.1086/5150389868662 [Google Scholar] [CrossRef] [PubMed]
[13]. Working Party of the British Society for Antimicrobial ChemotherapyAntibiotic treatment of streptococcal, enterococcal, and staphylococcal endocarditisHeart 1998 79(2):207-08.10.1136/hrt.79.2.207 [Google Scholar] [CrossRef]
[14]. Nishimura RA, Carabello BA, Faxon DP, Freed MD, Lytle BW, O’gara PT, ACC/AHA 2008 Guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic SurgeonsJ Am Coll Cardiol 2008 52(8):676-85.10.1016/j.jacc.2008.05.00818702976 [Google Scholar] [CrossRef] [PubMed]
[15]. Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009) The Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and by the International Society of Chemotherapy (ISC) for Infection and CancerEur Heart J 2009 30(19):2369-413. [Google Scholar]
[16]. Baddour LM, Wilson WR, Bayer AS, Fowler VG, Bolger AF, Levison ME, Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of AmericaCirculation 2005 111(23):394-434.10.1161/CIRCULATIONAHA.105.165563 [Google Scholar] [CrossRef]
[17]. Ako J, Ikari Y, Hatori M, Hara K, Ouchi Y, Changing spectrum of infective endocarditisCirculation 2003 67(1):3-7.10.1253/circj.67.3 [Google Scholar] [CrossRef]
[18]. Habib G, Derumeaux G, Avierinos JF, Casalta JP, Jamal F, Volot F, Value and limitations of the Duke criteria for the diagnosis of infective endocarditisJ Am Coll Cardiol 1999 33(7):2023-29.10.1016/S0735-1097(99)00116-3 [Google Scholar] [CrossRef]
[19]. Khaled AA, Al-Noami AY, Al-Ansi M, Faiza AA, Clinical features and outcome of infective endocarditis in Yemeni patients treated with empirical antibiotic therapyHeart Views: The Official Journal of the Gulf Heart Association 2010 11(1):2 [Google Scholar]
[20]. Tariq M, Siddiqui BK, Jadoon A, Alam M, Khan SA, Atiq M, Clinical profile and outcome of infective endocarditis at the Aga Khan University HospitalInt J Collab Res Intern Med Public Health 2009 1(3):84 [Google Scholar]
[21]. Strom BL, Abrutyn E, Berlin JA, Kinman JL, Feldman RS, Stolley PD, Risk factors for infective endocarditis: Oral hygiene and nondental exposuresCirculation 2000 102(23):2842-48.10.1161/01.CIR.102.23.2842 [Google Scholar] [CrossRef]
[22]. Oliver R, Roberts GJ, Hooper L, Worthington HV, Antibiotics for the prophylaxis of bacterial endocarditis in dentistryCochrane Database Syst Rev 2008 :410.1002/14651858.CD003813.pub3 [Google Scholar] [CrossRef]
[23]. Braun S, Escalona A, Chamorro G, Corbalán R, Pérez C, Labarca J, Infective endocarditis: Short and long-term results in 261 cases managed by a multidisciplinary approachRev Med Chil 2000 128(7):708-20.10.4067/S0034-98872000000700002 [Google Scholar] [CrossRef]
[24]. Ferreiros E, Nacinovich F, Casabé JH, Modenesi JC, Swieszkowski S, Cortes C, Epidemiologic, clinical, and microbiologic profile of infective endocarditis in Argentina: A national survey. The Endocarditis Infecciosa en la República Argentina–2 (EIRA-2) StudyAm Heart J 2006 151(2):545-52.10.1016/j.ahj.2005.04.00816442929 [Google Scholar] [CrossRef] [PubMed]
[25]. Jain SR, Prajapati JS, Phasalkar MA, Roy BH, Jayram AA, Shah SR, Clinical spectrum of infective endocarditis in a tertiary care centre in western India: A prospective studyInt J Clin Med 2014 5(05):17710.4236/ijcm.2014.55031 [Google Scholar] [CrossRef]
[26]. Tuğcu A, Yildirimtürk O, Baytaroğlu C, Kurtoğlu H, Köse O, Sener M, Clinical spectrum, presentation, and risk factors for mortality in infective endocarditis: a review of 68 cases at a tertiary care center in TurkeyTurk Kardiyol Dern Ars 2009 37(1):9-18. [Google Scholar]
[27]. Kanafani ZA, Mahfouz TH, Kanj SS, Infective endocarditis at a tertiary care centre in Lebanon: Predominance of streptococcal infectionJournal of Infection 2002 45(3):152-59.doi.org/10.1053/jinf.2002.1041 [Google Scholar] [CrossRef]
[28]. Faheem M, Iqbal MA, Saeed R, Asghar M, Hafizullah M, Profile of infective endocarditis in a tertiary care hospitalPakistan Heart Journal 2014 47(1) [Google Scholar]
[29]. Nunes MC, Gelape CL, Ferrari TC, Profile of infective endocarditis at a tertiary care center in Brazil during a seven-year period: prognostic factors and in-hospital outcomeInt J Infect Dis 2010 14(5):394-98.10.1016/j.ijid.2009.06.02419800277 [Google Scholar] [CrossRef] [PubMed]
[30]. Tsang TS, Barnes ME, Gersh BJ, Hayes SN, Risks of coronary heart disease in women: Current understanding and evolving conceptsIn Mayo Clinic Proceedings 2000 75(12):1289-303.10.4065/75.12.128911126839 [Google Scholar] [CrossRef] [PubMed]
[31]. Garg N, Kandpal B, Garg N, Tewari S, Kapoor A, Goel P, Characteristics of infective endocarditis in a developing country-clinical profile and outcome in 192 Indian patients, 1992-2001Int J Cardiol 2005 98(2):253-60.10.1016/j.ijcard.2003.10.04315686775 [Google Scholar] [CrossRef] [PubMed]
[32]. Duval X, Alla F, Doco-Lecompte T, Le Moing V, Delahaye F, Mainardi JL, Diabetes mellitus and infective endocarditis: the insulin factor in patient morbidity and mortalityEur Heart J 2006 28(1):59-64.10.1093/eurheartj/ehl31817040927 [Google Scholar] [CrossRef] [PubMed]
[33]. Thuny F, Grisoli D, Collart F, Habib G, Raoult D, Management of infective endocarditis: challenges and perspectivesThe Lancet 2012 379(9819):965-75.10.1016/S0140-6736(11)60755-1 [Google Scholar] [CrossRef]
[34]. Cheng A, Athan E, Appelbe A, McDonald M, The changing profile of bacterial endocarditis as seen at an Australian provincial centreHeart lung Circ 2002 11(1):26-31.10.1046/j.1444-2892.2002.00108.x16352065 [Google Scholar] [CrossRef] [PubMed]
[35]. Shashikala S, Kavitha R, Prakash K, Chithra J, Shailaja TS, Shamsul PM, Kocuria varians infective endocarditisInternet J Microbiol 2008 5(2)10.5580/4a5 [Google Scholar] [CrossRef]
[36]. Sanfilippo AJ, Picard MH, Newell JB, Davidoff R, Thomas JD, Echocardiographic assessment of patients with infectious endocarditis: prediction of risk for complicationsJ Am Coll Cardiol 1991 18:1191-99.10.1016/0735-1097(91)90535-H [Google Scholar] [CrossRef]
[37]. Fowler VG Jr, Sanders LL, Kong LK, McClelland RS, Gottlieb GS, Li J, Infective endocarditis due to staphylococcus aureus: 59 prospectively identified cases with follow-upClin Infect Dis 1999 28:10610.1086/51507610028079 [Google Scholar] [CrossRef] [CrossRef]
[38]. Kupferwasser LI, Hafner G, Mohr-Kahaly S, Erbel R, Meyer J, Darius H, The presence of infection-related antiphospholipid antibodies in infective endocarditis determines a major risk factor for embolic eventsJ Am Coll Cardiol 1999 33:136510.1016/S0735-1097(99)00024-8 [Google Scholar] [CrossRef]
[39]. Betigeri VM, Kareem RA, Nair RS, Nair SG, Kamath P, Nair SK, Left ventricular posterior wall pseudoaneurysm: a rare sequela of mitral valve infective endocarditis in a chronic patient of HLA-B27 positive spondyloarthritisAnn Thorac Surg 2006 82(6):2294-96.10.1016/j.athoracsur.2006.02.01617126160 [Google Scholar] [CrossRef] [PubMed]
[40]. Krecki R, Drozdz J, Ibata G, Lipiec P, Ostrowski S, Kasprzak J, Clinical profile, prognosis and treatment of patients with infective endocarditis-a 14-year follow-up studyPol Arch Med Wew 2007 117(11/12):51210.20452/pamw.244 [Google Scholar] [CrossRef]