Infections are the most important and leading cause of morbidity and mortality among the patients admitted to ICU [1]. A nosocomial infection-also called “hospital acquired infection” can be defined as “an infection acquired in hospital by a patient who was admitted for a reason other than that infection” [2], as an infection occurring in a patient in a hospital or other healthcare facility in whom the infection was not present or incubating at the time of admission. This includes infections acquired in the hospital but appearing after discharge, and also occupational infections among staff of the facility [3]. Infections acquired in health care settings are among the major causes of death and increased morbidity among hospitalised patients. With increasing infections, there is an increase in prolonged hospital stay, long-term disability, increased antimicrobial resistance, increase in socioeconomic disturbance, and increased mortality rate. Organisms causing nosocomial infections can be transmitted to the community through discharged patients, staff, and visitors. If organisms are multi drug resistant, they may cause significant disease in the community.
The most frequent types of infections include central line associated Blood stream infections, catheter-associated urinary tract infections, surgical site infections and ventilator-associated pneumonia [4]. Many different pathogens may cause nosocomial infections. The infecting organisms vary among different patient populations, health care settings, facilities, and countries. Pathogenic bacteria have greater virulence and cause infections (sporadic or epidemic) regardless of host status. These include Anaerobic Gram-positive rods (e.g., Clostridium), Gram-positive bacteria like Staphylococcus aureus (cutaneous bacteria that colonise the skin and nose of both hospital staff and patients) Gram-negative bacteria like Enterobacteriacae (e.g., Escherichia coli, Proteus, Klebsiella, Enterobacter, Serratia marcescens). Pseudomonas spp. is often isolated in water and damps. They may colonize the digestive tract of hospitalised patients. Selected other bacteria are a unique risk in hospitals, for instance, Legionella species may cause pneumonia (sporadic or endemic) through inhalation of aerosols containing contaminated water (air conditioning, showers, therapeutic aerosols) [3]. There is the possibility of nosocomial transmission of many viruses, including the hepatitis B and C viruses, Respiratory Syncytial Virus (RSV), rotavirus and enteroviruses (transmitted by hand-to-mouth contact and via the feco-oral route). Many fungi and other parasites are opportunistic organisms and cause infections during extended antibiotic treatment and severe immunosuppression (Candida albicans, Aspergillus spp., Cryptococcus neoformans, Cryptosporidium).
Bacterial colonisation of the pharynx and upper respiratory tract is initial portal of entry into normally sterile lower respiratory tract. Impaired physiological defence mechanisms including the cough reflex and innate immune system can predispose the airway to microbial invasion [5]. Colonisation is enhanced by therapeutic measures like ET incubation and suction [6]. The surfaces of an ET tube provide bacteria with a substratum that promotes microbial colonisation and biofilm formation. The formation of biofilm around the ET tubes by the micro-organisms [7] and their subsequent dislodgement following ET suction and repeated incubations contributes to lung colonisation and may lead ultimately to Ventilator Associated Pneumonia (VAP). Similar pathogens were isolated from ET tube biofilms and from cultures following ventilator associated pneumonia, which associates the biofilm presence on the ET tubes with infection.
Empirical antimicrobial therapy must be based on careful clinical evaluation and local epidemiological data regarding potential pathogens and antibiotic susceptibility. Appropriate specimens for Gram stain, culture and, if available, sensitivity testing must be obtained before starting therapy. In most ICUs, effective empiric therapy will require activity against Gram-negative bacilli, especially Pseudomonas and Acinetobacter, as well as Gram-positive organisms. If P. aeruginosa is the target organism, β-lactam antibiotics like Penicillins-Piperacillin; the third-generation Cephalosporins-Ceftazidime and Cefaperazone Sulbactam; the fourth-generation cephalosporin-Cefepime; the carbapenems-Imipenem, and Meropenem; the monobactam-Aztreonam (which can be used in the penicillin-allergic patient); and the β-lactam/β-lactamase inhibitor combinations can be used for optimal results. In patients with suspected methicillin resistant staphylococcal pneumonia, therapy should be with either Vancomycin or Linezolid as Tigecycline though as efficacious against Methicillin Resistant Staphylococcus Aureus (MRSA) is not efficacious in ventilator associated pneumonia. Acinetobacter spps. are inherently resistant to cephalosporins, Piperacillin-tazobactam and aminoglycosides and as resistance to carbapenems is more common, Polymyxin B and Colistin are frequently used. Drugs that were used to treat deadly diseases are now losing their impact due to emerging drug-resistant microorganisms. Self medication with antibiotics, incorrect dosage, prolonged use and lack of standards for healthcare workers is the main factors responsible for increase in resistance.
Against this background, authors attempted to study the time trends of bacterial colonisation of endotracheal tubes and the antibiotic sensitivities of the isolates in patients on mechanical ventilation for more than seven days.
Materials and Methods
A prospective observational study was done on 109 consecutive patients who got admitted and stayed mechanically ventilated for more than seven days. The study period was one year (April 2015 to March 2016) in ICUs (medical and respiratory) of a Tertiary Care Hospital in Southern India. The study was approved by the Institutional Ethical Committee (IEC No: 434/dt.09.04.2015).
Patients between the ages of 18 to 60 years were included in the study. Those patients who were intubated elsewhere and shifted to ICU were excluded from the study. HIV seropositive patients, pregnant and lactating women, patients with clinical suspicion of pre-existing sepsis, patients on steroid therapy and those patients/responsible attendants who did not give consent were also excluded from the study.
The ventilated patients were managed as per the ICU protocol, weaned off and extubated after they satisfied the extubation criteria. Relevant investigations were done as per the hospitals ICU protocol. Patients were monitored by BENEVIEW T8 PATIENT MONITOR (Shenzhen Mindray Bio-medical Electronics Co. Ltd., Shenzhen, China). Non-Invasive Blood Pressure, Oxygen Saturation, ECG, Heart Rate, and Respiratory Rate were recorded.
ET suction catheter tip was sent for bacterial culture at 24 hours and 48 hours post incubation and ET tube was removed and the tip was cut and sent for culture on 7th day of incubation. Tracheal end of the ET tube and ET suction catheter were cultured by removing the ET tube (using sterile technique) and severing 5 cm (2 inchs) segment from the distal tip of ET tube. The ET tube tip was placed in a sterile bottle for transport to the microbiology laboratory. For culture of the catheter tip, the roll-plate technique was used and 15 or more colonies were taken as significant. This was followed by vortexing of the catheter tip in phosphate buffered saline followed by plating to isolate the intraluminal colonising organisms and >103 Colony Forming Units (CFU)/mL of the vortex specimen was taken as significant. The samples were cultured on MacConkey’s agar and Blood agar and incubated for 24 hours at 37°C. After 24 hours, shape and colour of colonies and Gram-stain and sensitivity profile were studied. Bacterial strain identification was done by methods described previously [8]. Antibiotic sensitivity was done by disc diffusion technique as per Clinical and Laboratory Standards Institute (CLSI) guidelines [9].
The primary outcome measured was the time trend of bacterial colonisation of ET tube and sensitivity of colonised bacteria to the commonly used antibiotics.
Statistical Analysis
Data were recorded on a pre-designed proforma and managed using Microsoft Excel 2010 (Microsoft Corp, Redmond, USA). All entries were double checked for possible error. Categorical variables were summarised as percentages. Statistical analysis was done using SPSS 19 software.
Results
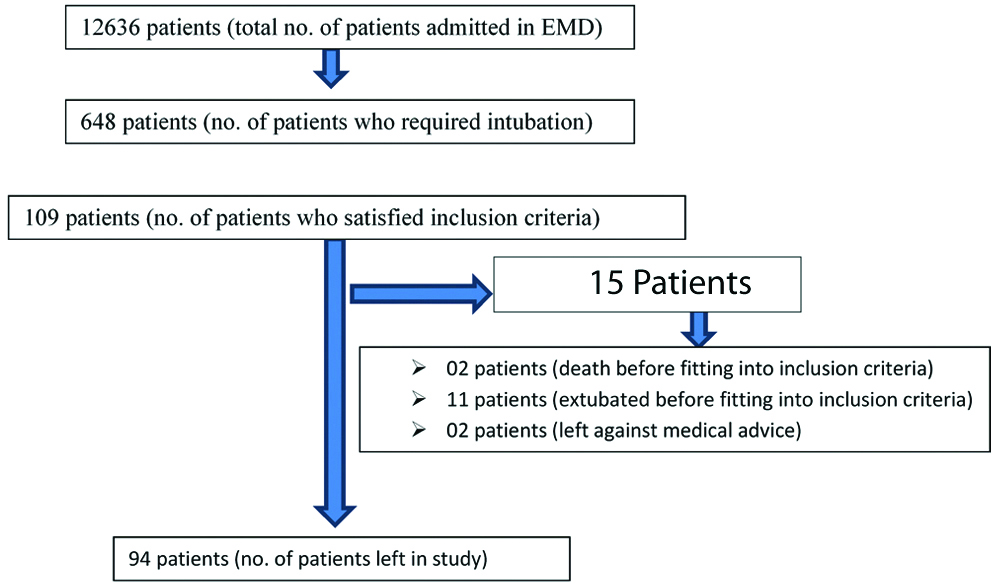
During the study period, a total of 12,636 patients required admission in the emergency room at the present institute. Of these, 648 patients were admitted in the Medical and Respiratory intensive care units and 350 required assisted mechanical ventilation. Authors studied a total of 109 subjects (who satisfied the inclusion criteria) from Medical and Respiratory intensive care Units. A total of 94 adult subjects were included, who were on mechanical ventilation for up to or more than seven days. Fifteen patients were excluded from the study (2 cases–death; 11 cases–extubation; 2 cases–LAMA (Left Against Medical Advice) before fitting into our criteria) [Table/Fig-1].
The bacterial colonisation of ET tubes, as measured by culture of ET suction catheters on 24 hours and 48 hours of incubation and of ET tube tip following 7th day of incubation showed an increasing trend of colonisation. 60 out of 94 patients at 24 hours (63.83%) and 76 out of 94 patients’ (80.85%) ET suction catheter tips at 48 hours were found to be colonised with micro-organisms. A total of 82 out of 94 patients (87.23%) of ET tube tip cultures done on 7th day of incubation were found to be colonised. Pseudomonas aeruginosa, Non Fermentative Gram Negative Bacilli (NFGNB) (which include Acinetobacter spp.) and Klebsiella pneumoniae were the predominant species isolated from ET suction catheter tip/tube at 24 hours [Table/Fig-2], 48 hours [Table/Fig-3] and on 7th day post incubation [Table/Fig-4] respectively.
Endotracheal suction catheter tip culture-at 24 hours post incubtion.
| Organism Isolated | Number (%)* |
|---|
| Non fermentative Gram negative bacilli including Acinetobacter | 17 (19.32) |
| Pseudomonas aeruginosa | 16 (18.18) |
| Klebsiella pneumonia | 12 (13.64) |
| Staphylococcus aureus | 11 (12.5) |
| Enterobacter spp. | 08 (9.09) |
| Enterococcus faecalis/bovis/faecium | 04 (4.55) |
| Citrobacter spp. | 04 (4.55) |
| Escherichia coli | 05 (5.68) |
| Coagulase negative staphylococci | 04 (4.55) |
| Non hemolytic Streptococci | 02 (2.27) |
| Candida non albicans | 02 (2.27) |
| Moraxella species | 01 (1.14) |
| Beta hemolytic streptococci | 01 (1.14) |
| Providentia species | 01 (1.14) |
| Total number of microorganisms isolated in 60 patients at 24 hours | 88 |
*=number of particular bacteria isolated/total number of bacteria isolated x100
Endotracheal suction catheter tip culture-at 48 hours post incubtion.
| Organism isolated | No. (%)* |
|---|
| Non fermentative Gram negative bacilli including Acinetobacter | 23 (20.35) |
| Pseudomonas aeruginosa | 21 (18.58) |
| Klebsiella pneumonia | 14 (12.39) |
| Enterobacter species | 12 (10.62) |
| Escherichia coli | 11 (9.73) |
| Coagulase negative Staphylococci | 9 (7.96) |
| Staphylococcus aureus | 7 (6.19) |
| Citrobacter species | 7 (6.19) |
| Enterococcus faecalis/bovis/faecium | 3 (2.65) |
| Proteus species | 2 (1.77) |
| Candida albicans | 2 (1.77) |
| Beta haemolytic Streptococci | 1 (0.88) |
| Candida non albicans | 1 (0.88) |
| Total number of microorganisms isolated in 76 patients at 48 hours | 113 |
*=number of particular bacteria isolated/total number of bacteria isolated x100
Endotracheal tube culture-7th day of incubation.
| Organism isolated | No. (%) |
|---|
| Pseudomonas aeruginosa | 29 (21.01) |
| Non fermentative Gram negative bacilli including Acinetobacter | 26 (18.84) |
| Klebsiella pneumonia | 26 (18.84) |
| Citrobacter species | 19 (13.77) |
| Escherichia coli | 16 (11.59) |
| Enterobacter species | 10 (7.25) |
| Enterococcus faecalis/bovis/faecium | 4 (2.90) |
| Staphylococcus aureus | 3 (2.17) |
| Proteus species | 2 (1.45) |
| Coagulase negative Staphylococci | 2 (1.45) |
| Beta haemolytic Streptococci | 1 (0.72) |
| Total no. of micro-organism isolates in 82 patients on seventh day of intubation=138 |
*=number of particular bacteria isolated/total number of bacteria isolated x100.
Eight out of seventeen patients (47.06%) of NFGNB including Acinetobacter and 14 out of 16 patients (87.5%) of Pseudomonas positive patients were sensitive to Cefaperazone-sulbactam and with Imipenem, the figures were 6 out of 17 patients (35.29%) for NFGNB and 15 out of 16 patients (93.57%) for Pseudomonas aeruginosa in 24 hour suction catheter tip culture. Almost similar trends were found for these isolates in 48 hours and 7th day cultures. The NFGNB including the Acinetobacter exhibited high frequency of resistance to the antibiotics which are in common use in ICUs. In contrast, P.aeruginosa showed less resistance to these antimicrobial agents [Table/Fig-5].
Antibiotic sensitivity pattern of non fermentative gram negative bacilli (including Acinetobacter) and Pseudomonas Isolates to 1st line anti microbial agents.
| Antibiotic | 24 hours% (No.) | 48 hours% (No.) | 7th day% (No.) |
|---|
| NFGNB-17 | Pseudomonas-16 | NFGNB-23 | Pseudomonas-21 | NFGNB-26 | Pseudomonas-29 |
|---|
| Amikacin | 35.3 (6) | 87.50 (14) | 34.78 (8) | 71.43 (15) | 34.62 (9) | 75.86 (22) |
| Amoxyclav | 11.76 (2) | - | 13.04 (3) | - | 3.85 (1) | - |
| Ampicillin | 17.65 (3) | - | 13.04 (3) | - | 3.85 (1) | - |
| Cefeperazone-sulbactam | 47.06 (8) | 87.50 (14) | 69.57 (16) | 85.71 (18) | 46.15 (12) | 86.21 (25) |
| Cefotaxime | 11.76 (2) | 50 (8) | 13.04 (3) | 66.67 (14) | 7.69 (2) | 34.48 (10) |
| Imipenem | 35.29 (6) | 93.75 (15) | 34.78 (8) | 95.24 (20) | 38.46 (10) | 82.75 (24) |
| Piperacillin-Tazobactam | 25.53 (4) | 81.25 (13) | 30.43 (7) | 76.19 (16) | 15.38 (4) | 75.86 (22) |
In the present study, 8 out of 94 patients developed VAP. Klebsiella pneumoniae was the commonest organism isolated (n=5) followed by Pseudomonas aeruginosa and NFGNB including Acinetobacter (n=3 each), and Citrobacter spp. isolated in one instance [Table/Fig-6].
Causative agents of ventilator associated pneumonia.
| Causes | Number of positive cultures |
|---|
| Polymicrobial | 03 |
| Klebsiella pneumoniae + Pseudomonas aeruginosa | 05 |
| Non fermentative gram negative bacilli including Acinetobacter | 03 |
| citrobacter | 01 |
Variable pattern of sensitivity was observed for the different organisms to the commonly used antibiotics used in ICU settings and no single antibiotic showed superior efficacy.
Discussion
A cross-sectional observational study was done on 94 subjects who were mechanically ventilated in medical and respiratory intensive care units for more than seven days. The study was intended to evaluate the time trends of bacterial colonisation inside the ET Tubes (ETT) and antibiotic sensitivity profile of colonised bacteria in patients receiving assisted mechanical ventilation for more than seven days in medical and respiratory ICUs of a tertiary care teaching hospital in Southern India from April 2015 to March 2016.
Sixty out of ninty-four patients (63.83%) of the tracheal aspirate specimens had bacterial colonisation at 24 hours. The colonisation rate increased to 76 out of 94 patients (80.85%) at 48 hours post incubation and then to 87.23% (82 out of 94 patients) by 7th day of incubation. There has been a steady increase in the bacterial colonisation as the number of days on ventilator increased.
ET tube suction catheter tip culture at 24 hours and at 48 hours showed a preponderance of NFGNB including Acinetobacter which was isolated in 19.32% (17 out of 88 isolates) and 20.35% cases (23 out of 113 isolates) respectively. Pseudomonas aeruginosa and Klebsiella pneumoniae isolation rates remained almost constant during the same said time period. Enterobacter spp. and Escherichia coli isolates increased at 48 hours compared to 24 hours specimen (10.62% and 9.09% respectively for Enterobacter spp, 9.73% and 5.68% for Escherichia coli). In the 7th day ET tube tip culture, Pseudomonas aeruginosa was isolated in 21.01% of cultures (29 out of 138 positive cultures) followed by NFGNB including Acinetobacter and Klebsiella pneumonia (18.84% of 138 positive cultures) and Escherichia coli (11.59% of 138 positive cultures) [Table/Fig-2,3 and 4].
Twenty-four hours tracheal aspirate of NFGNB including Acinetobacter isolates showed maximum sensitivity to Cefaperazone-sulbactam (47%). The sensitivity increased to 69.57% in the 48 hours culture. Pseudomonas aeruginos showed maximum sensitivity to Imipenem which was observed in 93.7% at 24 hours and 95.24% of isolates at 48 hours. An 87.5% of cultures were sensitive to Cefaperazone-sulbactam. Only 50% of Pseudomonas aeruginosa isolates were sensitive to cefotaxime in first 24 hours of incubation, which dropped down to nearly 34.48% on the 7th day [Table/Fig-4].
VAP developed in 8 of 94 patients (8.51%) [Table/Fig-6]. Only one patient had VAP five days after incubation and two patients had VAP eight days after incubation. Six out of eight patients succumbed to death because of VAP.
Almost 50% isolates of NFGNB including Acinetobacter showed resistance to commonly used antibiotics in ICUs at all time points of study. The exception was the cefaperazone-sulbactam combination [Table/Fig-5].
In a study by Inglis TJ et al., they isolated gram-negative bacilli in a progressively increasing proportion at successive sampling points [10]. This observation was found to be consistent with endogenous to external route of spread of bacteria. Present study is also concordant with above study in that isolation of Gram-negative bacilli progressively increased from 63.83% in the first sample to 87.23% in 7th day sample. Ewig S et al., found that initial colonisation was mainly by Group 1 pathogens like Streptococcus viridans, Staphylococcus aureus, and Haemophilus influenza [11]. Later, frequency of colonisation with Group 2 pathogens i.e., Gram negative enteric bacilli and Pseudomonas increased. Present study largely corroborated the findings of the above study in that at 24 hours and 48 hours sample collection, the most common isolate was NFGNB including Acinetobacter and on 7th day, Pseudomonas aeruginosa. However, the Gram positive isolates in the present study were much fewer in number compared to Gram negative bacteria, which is in contrast to the above study [11]. This difference may be due to fact that in their study, they selected only medical/surgical head injury patients whereas present authors selected all non-intubated patients who were admitted to our ICU and later got intubated.
In a study done by Gil-Perotin S et al., all 60 (100%) of ET Aspirate (ETA) samples grew micro-organisms [12]. In Saha AK et al., study the incidence of growth positive ETA was 59.26% [13]. In the present study, growth positivity for ETA was 63.83% at 24 hours, 80.85% at 48 hours and 87.23% on 7th day of incubation. The variations among the various studies on incidence of growth positivity, including the present study, can be explained by the difference in the technique of incubation, clinical and individual characteristics of study population, colonisation during incubation or lack of sufficient precautions during incubation due to high work load in an emergency setting.
Acharya R et al., found that Pseudomonas aeruginosa (39.7%) was the commonest colonising organism isolated from ETA, followed by Acinetobacter (27.9%) and Staphylococci (19.1%) [14]. In Saha AS et al., study, Acinetobacter (33.34%) was the predominant organism isolated followed by Klebsiella (31.73%) [13]. In Hoque L et al., study, Acinetobacter was isolated in 25% of ETA, Pseudomonas in 15% followed by Klebsiella in 10% cases [15]. This is in line with the present study where the predominant organism at 24 hours and 48 hours was NFGNB including Acinetobacter (19.32% and 20.35% respectively) and on 7th day it was Pseudomonas (21.91%). The next common organism was Klebsiella having an incidence of 13.64%, 12.39% and 18.84% at 24 hours, 48 hours and on 7th day respectively. These findings are similar to the observations made in the previous two studies[13,15].
It is obvious from the different studies from various corners of the world that bacterial isolates differ not only in number but also in the frequency and order of isolation. This may be due to different factors like prevalence of resident and resistant flora, inadvertent use of antibiotics and different morbid factors like diabetes mellitus, use of immunosuppressive therapies, decreased immunity, and chronic diseases like chronic liver disease, interstitial pulmonary fibrosis, bronchial asthma, stroke, malignancies, and chronic renal failure [13].
In a study by Tullu MS et al., E.coli was the commonest organism isolated and it had maximum susceptibility to Cefotaxime and Amikacin [16]. On the other hand, an eastern Indian study found Klebsiella pneumonia and Pseudomonas aeruginosae being highly sensitive to Meropenem (90.9% and 100% respectively), while sensitivity to Imipenem was found in 88% of Escherichia coli isolates [13].
In the present study, 24 hours ET suction tip culture showed that 47.06% of NFGNB and 87.50% of Pseudomonas aeruginosa were sensitive to Cefaperazone-sulbactam. At 48 hours, the sensitivity frequency for these two groups of organisms was 69.57% and 85.71% respectively for that antibiotic. For imipenem, 35.29% of the NFGNB and 93.75% for Pseudomonas aeruginosa were sensitive at 24 hours and these figures remained almost unchanged at 48 hours (34.78% and 95.24% respectively). On 7th day, resistant strains started appearing and sensitivity to Cefaperazone-sulbactam dropped to 46.15% for NFGNB including Acinetobacter, and 86.21% for Pseudomonas aeruginosa. For Imipenem, 38.46% of NFGNB remained sensitive, while 82.75% of Pseudomonas aeruginosa strains were sensitive to that antibiotic [Table/Fig-4].
In Khosravi AD et al., study, Enterobacter was isolated in 41.4% of cases which was resistant to Imipenem in 59.3% of cases [17]. In present study, Enterobacter resistance to Imipenem was about 12.5% at 24 hours rising to 30%, on 7th day.
In a study done by Panda G et al., analysis of microbiological profile and sensitivity of ET aspirates of mechanically ventilated patients were made [18]. They found that Acinetobacter was highly sensitive to Polymyxin B, intermediately sensitive to imipenem and resistant to Cefaperazone-sulbactam and Piperacillin-Tazobactam antibiotics. Pseudomonas aeruginosa was extremely sensitive to Polymyxin and imipenem and resistant to Cefaperazone-sulbactam. Klebsiella species also showed highest degree of sensitivity to Polymyxin and resistance to ceftriaxone. Pravin Charles MVP et al., isolated Pseudomonas and Klebsiella which showed 100% sensitivity towards Imipenem and Pipericillin-Tazobactam [19]. Goel N et al., studied the antibiotic sensitivity of gram negative bacilli isolated from lower respiratory tract of patients on mechanical ventilation for more than 72 hours [20]. Their results revealed 77.2% sensitivity to Meropenem for pseudomonas species and a 40% resistance to all the antibiotics in the panel. The isolated Acinetobacter and klebsiella species were 60-100% resistant to ceftazidime. Golia S et al., isolated micro-organisms causing ventilator-associated pneumonia [21]. They found that all gram-positive cocci were sensitive to Linezolid and vancomycin. Pseudomonas showed 55% sensitivity to Pipearicillin-Tazobactam. Escherichia coli were 100% sensitive to ceftazidime and Acinetobacter was sensitive to piperacillin-Tazobactam in 75% of instances. In present study, Acinetobacter species were resistant to most of drugs on the antibiotic panel whereas the pseudomonas species showed maximal sensitivity to imipenem. The present study differs from above studies in that authors isolated microorganisms at three time points which explain the decrease in antibiotic sensitivity with passage of time [Table/Fig-5]. Also, the aetiological agents causing infection varies according to intensive care unit population, duration of hospital stay and prior antibiotic therapy. Administration of inadequate and inappropriate antibiotic treatment can enhance patient mortality.
In a study done by Gil-Perotin S et al., 19% of patients developed 17 episodes of VAP during their stay in ICU [12]. Early onset VAP (less than four days of incubation) was caused by Haemophilus influenzae, Enterobacter and Streptococcus species. Late onset VAP was caused by Acinetobacter in 54% of subjects and Pseudomonas in 27% of patients. Days of incubation and previous airway colonisation by Pseudomonas and Acinetobacter were associated with development of VAP [13]. In the study done by Acharya R et al., in Nepal, 10% (3 patients) of tracheostomised patients developed VAP and most common causative organism of VAP was Pseudomonas [14]. In our study, 8.51% (8 patients) of 94 patients developed VAP. A total of eight episodes of VAP were observed (incidence rate of 6.91 episodes/1000 mechanical ventilator days). The earliest incidence was five days in one patient, 8th day in two patients. In one patient, VAP developed after 38 days of incubation. In the present series, out of four patients who developed VAP within 10 days of incubation, Klebsiella and Pseudomonas were isolated in three patients and NFGNB isolated in one patient. This is similar to the findings of study done by Gil-Perotin S et al., where they found that Acinetobacter baumannii was responsible for 54% of late onset pneumonia [12]. Despite the high prevalence of airway colonisation on ETT (1st-7th day), only 8.51% of present patients developed VAP in present study group. Therefore, author opine that airway colonisation was necessary but not enough to explain the development of VAP. Seven out of eight patients in present group developed VAP after 8 days of incubation. So, prolonged hospitalisation and prolonged mechanical ventilation play a role in development of VAP. There was concordance between bacterial colonisation of the airway (7th day of ET tip culture) and VAP causing organisms in 50% of these patients.
The present study tells us the importance of knowing the pathogens and their antibacterial susceptibility pattern, prevalent in the particular ICU, to initiate the empirical antibacterial therapy for patients on mechanical ventilation. This study also tells us the need for a proper empirical antimicrobial therapy protocol in each hospital based on the most common microorganism isolated and its antibiotic sensitivity. This policy should be drawn by all the stake-holders in the treatment-general physicians, intensivists and microbiologists.
Limitation
Two important limitations of the study need to be mentioned. First, present cases were few in number because the hospital is a tertiary care health centre catering to the needs of four districts in Rayalaseema region of Andhra Pradesh state and patients who come for admission here would have been already intubated at other centres or having pre-existing septic foci or may be already under antibiotic coverage. Secondly, a more comprehensive picture would have emerged if authors had studied all the intubated patients in other ICUs in our hospital.
Conclusion
ET Aspirate culture provides information in instituting early treatment protocol which should be made on the basis of antimicrobial profile to prevent mortality in Ventilator Associated Pneumonia (VAP) patients.
Endotracheal aspirate culture provides information in instituting early treatment protocol which should be made on the basis of antimicrobial profile to prevent mortality in VAP patients. In the present study, authors found Non Fermentative Gram Negative Bacilli (NFGNB) including Acinetobacter, Pseudomonas aeruginosa and Klebsiella pneumoniae to be the most frequent colonisers in mechanically ventilated patients during the first week of incubation. Most of these isolates were sensitive to cefoperazone-sulbactam and Imipenem.
Further studies are needed; first, to clarify the exact role of ET colonisation in the causation of ventilator-associated pneumonia; and secondly, similar work should be undertaken at other hospitals of this region to prepare a comprehensive regional database of ETA colonising bacteria and their antimicrobial resistance.
*=number of particular bacteria isolated/total number of bacteria isolated x100
*=number of particular bacteria isolated/total number of bacteria isolated x100
*=number of particular bacteria isolated/total number of bacteria isolated x100.