Case Report
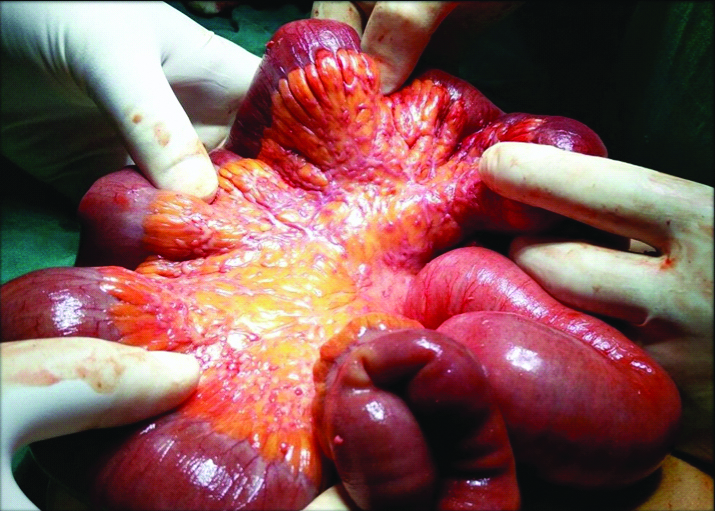
A 75-year-old female, a treated case of pulmonary tuberculosis presented with pain abdomen since one week and vomiting since day one at the Department of Surgery. Per abdominal examination revealed vague tenderness involving all the quadrants and vital signs included Pulse Rate: 98 beats/minute, BP: 100/70 mmHg, Respiratory Rate: 16 breaths/minute. Patient had a past history of pulmonary tuberculosis for which she was treated, detailed records of the same were not available with her. Clinically, a diagnosis of abdominal tuberculosis was made during this presentation. Differential diagnosis of acute cholangitis, acute cholecystitis, biliary colic, inflammatory bowel disease and endometriosis were also considered. The patient had CT scan abdomen done at another hospital, report showed bowel ischaemia involving a large segment of ileum along with atherosclerotic changes in the mesenteric blood vessels and images were not available with the patient. Following which an exploratory laparotomy was performed which revealed ileal stricture, ischaemia and multiple small nodules in mesentery. Hence, a tentative intraoperative diagnosis of tuberculosis was made. No other investigations were done. Ischaemic bowel along with mesentery was resected and sent for histopathological examination [Table/Fig-1].
Intraoperative picture showing ischaemic bowel along with multiple tiny nodules in the mesentry.
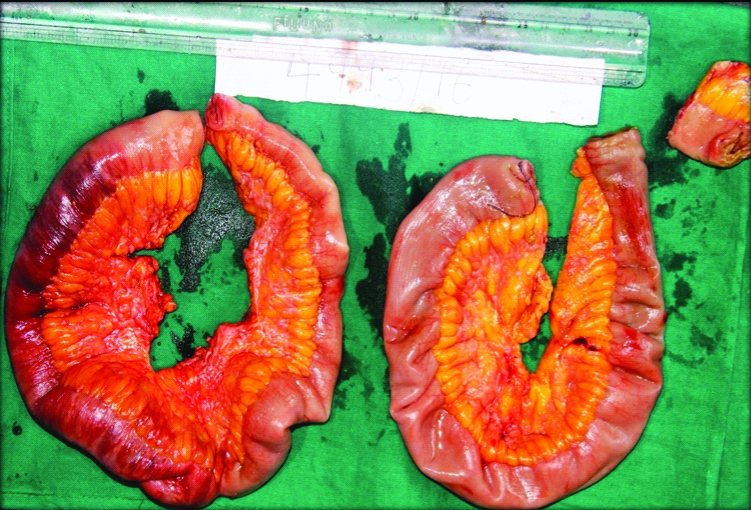
Grossly received three ileal segments with attached mesentery; longest segment measuring 48 cm and smallest measuring 2 cm in length. All the three segments had patchy blackish areas and the longest segment showed a stricture measuring 2 cm×2 cm. Mesentery of all the segments showed multiple tiny white nodules measuring 0.2 cm×0.2 cm to 0.5×0.5 cm. No lymph nodes were retrieved [Table/Fig-2].
Gross specimen of resected intestine showing focal blackish area and a stricture. Mesentry showing multiple tiny nodules.
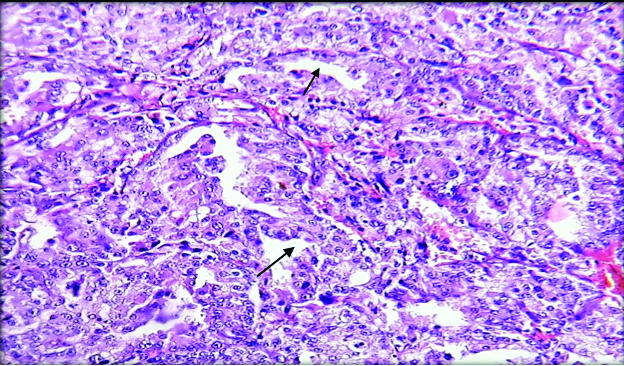
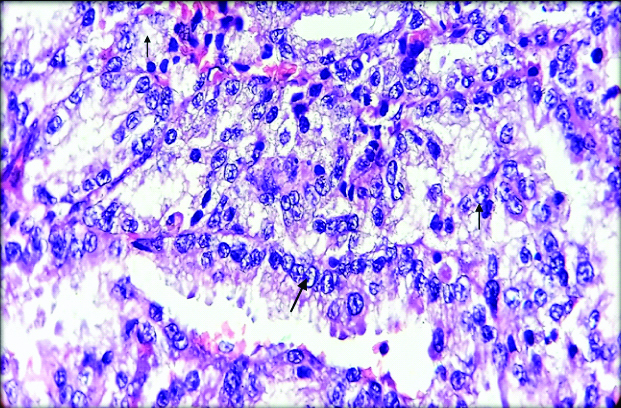
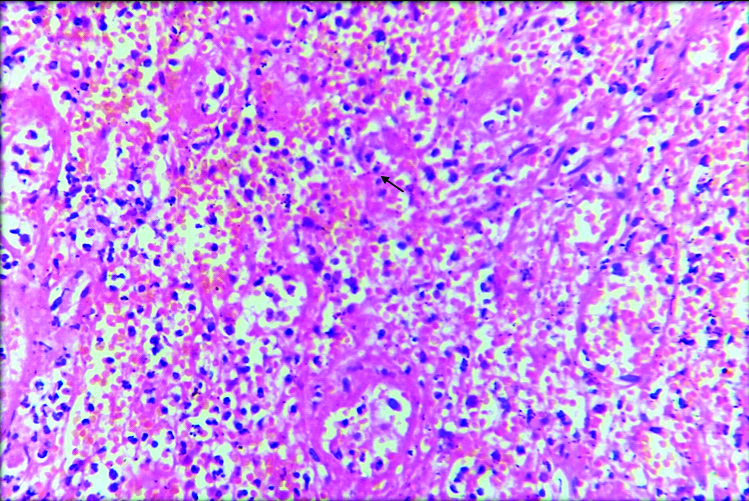
On microscopy, sections from mesenteric nodules showed tumour tissue arranged in trabecular and insular pattern. Individual tumour cells were round to oval having round to oval nucleus with regular nuclear membrane, salt and pepper chromatin and moderate amount of eosinophilic cytoplasm [Table/Fig-3,4]. 1-2 mitotic figures/10 HPF were also noted. No evidence of coagulative necrosis, Langhan type of giant cells and lymphovascular or perineural invasion. Histopathologically, features were in favour of neuroendocrine carcinoma.
Microphotograph shows section from the mesenteric nodule showing tumour tissue arranged in trabecular and insular pattern. (H&E, 100X)
Microphotograph from the section shows individual tumour cells are round to oval with round to oval nucleus having regular nuclear membrane with salt and pepper chromatin (arrow) and moderate cytoplasm (H&E, 400X).
Multiple sections from the stricture and ischaemic areas showed ulcerated mucosa with large areas of haemorrhage, dense inflammatory cell infiltrate and extensive areas of ischemic necrosis [Table/Fig-5]. Surgical margins were free of tumour tissue. In spite of extensive grossing, primary was not identified in the resected segment.
Microphotograph shows section from the stricture showing large area of ischemic necrosis along with dense inflammation. (H&E, 100X).
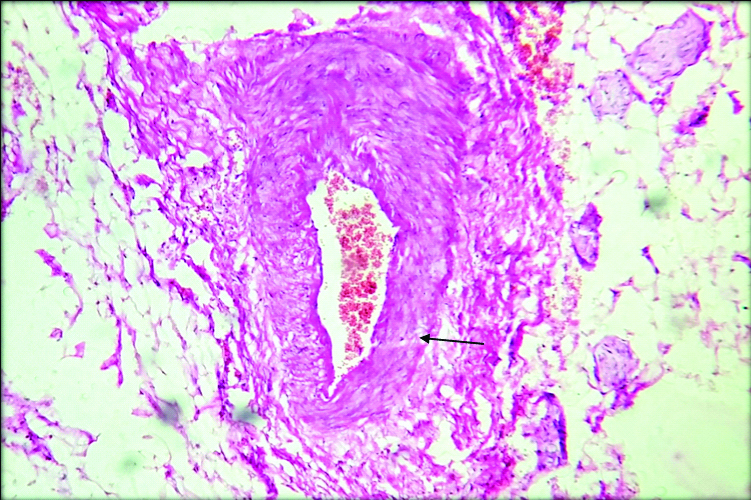
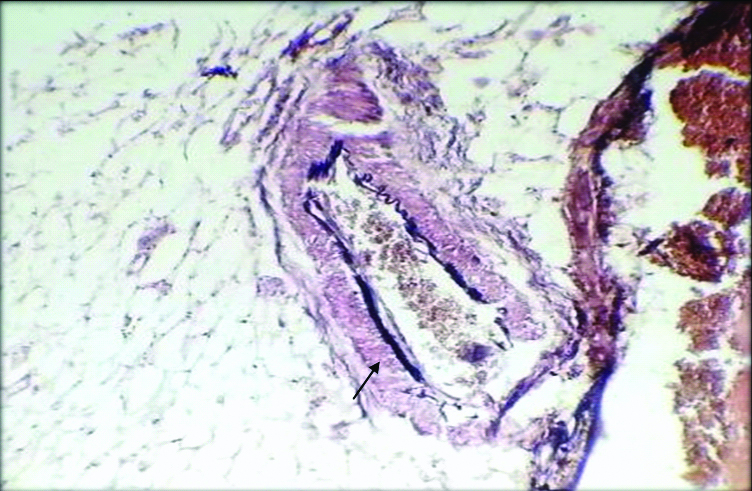
Sections from the mesentery also included mesenteric blood vessels which were thickened and showed perivascular connective tissue deposits [Table/Fig-6]. In the present case, Verhoff staining was done which demonstrated high content of elastic fibres in the adventitial connective tissue favouring the diagnosis of elastic vascular sclerosis of mesenteric vessels [Table/Fig-7].
Microphotograph shows section from the thickened mesenteric blood vessel showing perivascular fibrosis (H&E, 100X).
Microphotograph shows section from the thickened mesenteric blood vessel showing perivascular fibrosis (H&E, 100X).
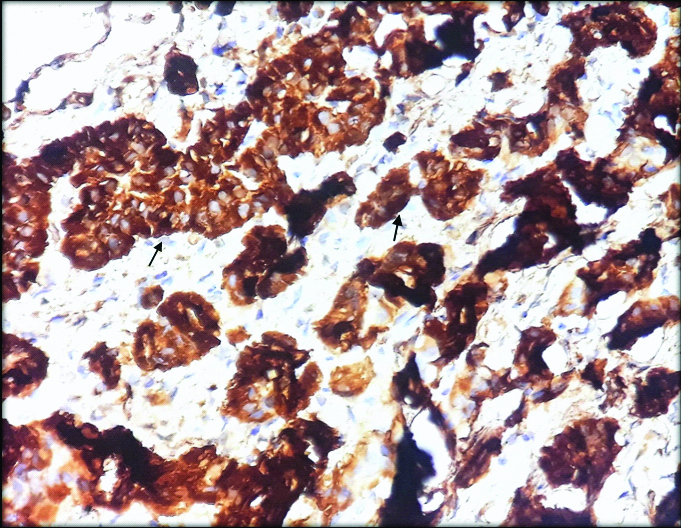
As present histopathological diagnosis was neuroendocrine carcinoma, immunohistochemistry (IHC) was performed with Chromogranin A which showed intense cytoplasmic positivity for Chromogranin A [Table/Fig-8]. Based on gross, histomorphological features and intense chromogranin positivity, diagnosis of metastatic well-differentiated neuroendocrine tumour in the mesentery was made. Staging of the tumour was not possible as the primary could not be identified. The need for further evaluation of primary tumour being in other organs like lung, liver and bone was suggested, patient was lost to follow-up.
Microphotograph of Tumour cells showing cytoplasmic positivity with chromogranin A (400X).
Discussion
Carcinoid tumours are rare tumours accounting for about 2% of all cancers, approximately 2.5 cases per 100,000 people [1]. These tumours arise from neuroendocrine cells most commonly in the gastrointestinal tract followed by the respiratory system [2]. These tumours are relatively slow growing and around 40-60% of the patients are asymptomatic at presentation. Gastric carcinoids account for < 1% of all gastric neoplasms but up to 6% of Gastrointestinal carcinoid tumors (GICTs). Carcinoid syndrome is typically seen in patients with liver/lung metastases with an overall incidence of 10% in GICTs but 20% in those with jejuno-ileal disease [3]. Hence, may be clinically apparent only after a metastatic spread or evidence of Carcinoid syndrome [1,2]. Rarely these tumours may cause ischaemic necrosis in the non-neoplastic small intestine due to elastic vascular sclerosis of the mesenteric vessels [4]. Here, authors present a case of metastatic mesenteric Carcinoid associated with ischaemic necrosis of the ileum.
Neuroendocrine Tumours (NETs) have a low incidence but relatively high prevalence. They are classified as either pancreatic endocrine tumours or Carcinoid tumours. In India, the estimated prevalence of NET is 1-2 cases per 100,000 people, of which gastrointestinal tract is the most common site and incidence of GI-NET is around 67.5% amongst all NET [5]. The incidence of NETs has risen 6-fold in the United States in the last three decades. It is diagnosed most commonly between the ages of 50-70 years. Carcinoid tumour prevalence appears to be slightly higher in African Americans with 4.48 and 3.98 cases per 100,000 African American men and women, respectively. According to recently published papers, increasing trends were also observed in China, Japan, Korea, Norway, Netherlands, and Taiwan [6,7].
Carcinoid tumours often present in 5th or 6th decade of life with slight male preponderance. Gastrointestinal tract is the common site for Carcinoid tumour followed by trachea-bronchopulmonary tree, kidney and ovary [8]. Carcinoid tumours in the GIT arise from neuroectodermal cells known as Kulchitsky cells. Small intestine is the most common primary site followed by appendix and rectum in the GIT [2,8].
The histopathological differential diagnosis of mesenteric carcinoid tumour includes the malignant tumours of the small intestine such as adenocarcinoma, lymphoma, GIST and metastasis [9]. Carcinoid tumours are known to produce and secrete endogenously a number of substances like NSE, 5-HT, synaptophysin, prostaglandins, gastrin, insulin and various growth factors such as platelet-derived growth factor, transforming growth factor and vascular endothelial growth factor [2,3].
The most common complications associated with Carcinoid syndrome are Carcinoid Heart Disease (CHD), characterised by a fibrotic degeneration of cardiac valves and extracardiac fibrosis [10]. Serotonin is thought to be responsible for the cardiac valvular fibrosis found in the Carcinoid syndrome [6].
About 5% of these patients may present with Carcinoid syndrome which occurs due to secretory activity of the tumour. The syndrome is often seen in patients with metastatic liver disease or when the primary site allows the secreted amines to escape enterohepatic circulation [10]. It results from interaction between 5HTP metabolites, kinins and prostaglandins and produces flushing, diarrhoea, abdominal cramps, heart- valve dysfunction. Typical presentation of GIT Carcinoids includes pain abdomen, diarrhoea, hypotension, gastrointestinal bleeding and obstruction [6]. Tumour-induced ischaemic necrosis in the non-neoplastic small intestine is a rare presentation of these tumours [4].
Various mechanisms have been proposed for the occurrence of tumour induced ischaemia. One of the hypothesis states that metastatic nodules caused obstruction to venous drainage of the loop resulting in ischaemia of the segment. Aetiological factor for vascular change is the tumour locally inducing vascular fibroblasts to produce an elastic tissue which cause kinking of the blood vessels [11].
While few authors postulated that vascular occlusion was the consequence of secretory activity of the tumour which resulted in obliterative elastic sclerosis of the mesenteric vessels. This entity is characterised by thick elastic tissue deposition in the adventitia of mesenteric arteries and veins [4,11,12]. Decreased blood flow due to decreased cardiac function or mesenteric arterial atherosclerosis could also predispose to tumour induced ischaemic necrosis [11]. In the present case, obliterative elastic sclerosis of mesenteric blood vessels is thought to be the etiological factor for ischaemic necrosis in the non-neoplastic ileal segment.
Potentially curative therapy for Carcinoid tumours is the wide surgical resection of the primary tumour and its lymphatic drainage. For tumours smaller than 2 cm in their greatest dimension and in the absence of lymph node metastases, an approach of local excision for incidental tumours of the stomach, rectum, and appendix has been successful. More radical surgery would be appropriate for tumours larger than 2 cm, with or without lymph node metastases. Because of the association of multifocal disease with other gastrointestinal tumours, a thorough exploration of the abdominal cavity is indicated [2].
In the present case, multiple mesenteric deposits of Carcinoid were noted. However, the primary was not identified in the resected ileal segment. Landau M et al., in their study mentioned that 40% (4 out of 10) of patients with tumour induced ischaemia had mesenteric tumour without a primary tumour being identified. They also mentioned that jejuno-ileal Carcinoid that is smaller than 1cm is likely to be missed intraoperatively [4].
The prognosis for patients with metastatic carcinoid tumours has improved during the last decade and has a good prognosis if discovered early before metastasis. Prognosis is related to site: those in the vermiform appendix having a five-year survival rate of 99% compared with 54% for those of small intestinal origin. Special attention should be given to unexpected metastatic patterns, like bone metastases. In metastatic carcinoid, patients may have better quality of life and longer survival times by combining new diagnostic and treatment modalities [6,13].
Conclusion
Carcinoid induced ischaemic necrosis pose a diagnostic difficulty clinically. Awareness of ischaemic enteritis as a complication of carcinoid is important for management of the patients, as they are associated with high mortality and an overall poor prognosis.
[1]. Yener O, Intestinal ischaemia associated with carcinoid tumour: a case report with review of the pathogenesisPrague Medical Report 2013 114:43-47.10.14712/23362936.2014.3823547726 [Google Scholar] [CrossRef] [PubMed]
[2]. Schnirer II, Yao JC, Ajani JA, Carcinoid-a comprehensive reviewActa Oncol 2003 42(7):672-92.10.1080/0284186031001054714690153 [Google Scholar] [CrossRef] [PubMed]
[3]. Dronamraju SS, Joypaul VB, Management of gastrointestinal carcinoid tumours-10 years experience at a district general hospitalJ Gastrointest Oncol 2012 3(2):120-29. [Google Scholar]
[4]. Landau M, Wisniewski S, Davison J, Jejunoileal neuroendocrine tumours complicated by intestinal ischemic necrosis are associated with worse overall survivalArch Pathol Lab Med 2016 140(5):461-66.10.5858/arpa.2015-0105-OA27128303 [Google Scholar] [CrossRef] [PubMed]
[5]. Amarapurkar DN, Juneja MP, Patel ND, Amarapurkar AD, Amarapurkar PD, A retrospective clinico-pathological analysis of neuroendocrine tumours of the gastrointestinal tractTrop Gastroenterol 2016 31(2):101-04. [Google Scholar]
[6]. Harvey JN, Denyer ME, DaCosta P, Intestinal infarction caused by carcinoid associated elastic vascular sclerosis: early presentation of a small ileal carcinoid tumourGut 1989 30(5):691-94.10.1136/gut.30.5.6912731764 [Google Scholar] [CrossRef] [PubMed]
[7]. Chauhan A, Yu Q, Ray N, Farooqui Z, Huang B, Durbin EB, Global burden of neuroendocrine tumours and changing incidence in KentuckyOncotarget 2018 9(27):19245-54.10.18632/oncotarget.2498329721198 [Google Scholar] [CrossRef] [PubMed]
[8]. Abdulsamad M, Abbas N, Balar B, An unusual case of rectal and ileal carcinoid tumoursCase Rep Gastroenterol 2016 10(3):793-99.10.1159/00045470828203126 [Google Scholar] [CrossRef] [PubMed]
[9]. Anzidei M, Napoli A, Zini C, Kirchin MA, Catalano C, Passariello R, Malignant tumours of the small intestine: a review of histopathology, multidetector CT and MRI aspectsBr J Radiol 2011 84(1004):677-90.10.1259/bjr/2067337921586504 [Google Scholar] [CrossRef] [PubMed]
[10]. Mota JM, Sousa LG, Riechelmann RP, Complications from carcinoid syndrome: review of the current evidenceEcancermedicalscience 2016 10:66210.3332/ecancer.2016.66227594907 [Google Scholar] [CrossRef] [PubMed]
[11]. Bessell JR, Karatassas A, Allen PW, Intestinal ischaemia associated with carcinoid tumour: a case report with review of the pathogenesisJ Gastroenterol Hepatol 1994 9(3):304-07.10.1111/j.1440-1746.1994.tb01730.x [Google Scholar] [CrossRef]
[12]. Chapuis P, Weedon D, Ischaemic ileal necrosis and carcinoid tumourAust N Z J Surg 1976 46(1):63-64.10.1111/j.1445-2197.1976.tb03196.x1064406 [Google Scholar] [CrossRef] [PubMed]
[13]. Zuetenhorst JM, Taal BG, Metastatic carcinoid tumours: a clinical reviewOncologist 2005 10(2):123-31.10.1634/theoncologist.10-2-12315709214 [Google Scholar] [CrossRef] [PubMed]