A 12-year-old boy suddenly collapsed while playing and was brought to the emergency department, where he was declared dead. The child had no prior medical illness. On autopsy examination, a dumbbell shaped mass was found in the right atrioventricular sulcus on the anterior and posterior aspects. Microscopic examination of the mass revealed a thrombus within the wall of right coronary artery. Careful examination of the epicardial tissue close to the thrombus revealed a granulomatous vasculitis of the right coronary artery, with involvement of the root of aorta. A possible diagnosis of Takayasu’s arteritis was rendered. Vasculitis is the inflammation of the blood vessels. Kawasaki’s and Takayasu’s arteritis are the most common ones affecting the vessels of the heart. Takayasu’s arteritis or “pulseless” disease is a rare, idiopathic, chronic granulomatous vasculitis that affects aorta and its major branches. The rarity of its occurrence and clinical presentation as a thrombus masquerading as cardiac tumour is unusual and hence reported.
Paediatric vasculitis, Sudden cardiac death, Takayasu’s arteritis
Case Report
A 12-year-old male child was brought to the emergency medicine department. He had collapsed while playing and was declared dead at the hospital. As the child had no previous medical illnesses/drug history, he was subjected to a medico legal autopsy, performed by the Department of Forensic Medicine. No external injuries were identified.
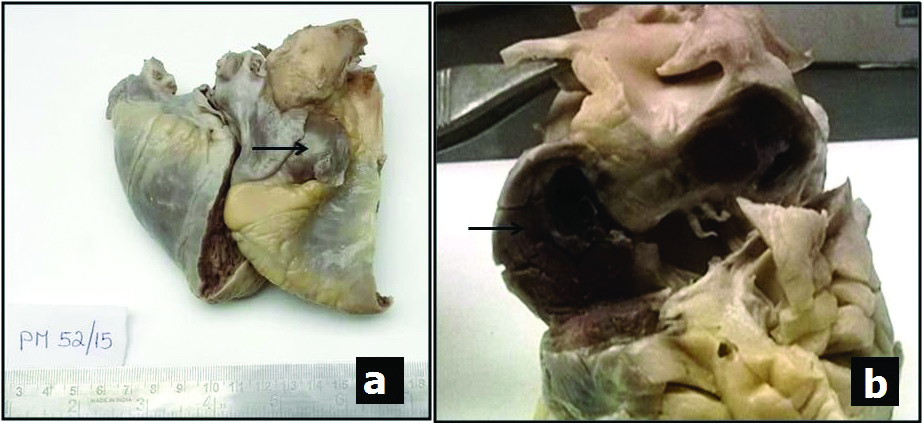
The internal organs were dissected and various organs including the heart, liver, lungs, spleen and kidney were received in the Department of Pathology for histopathological examination. All organs were received intact with unremarkable macroscopic examination except the heart. The heart weighed 178g and showed a dumbbell shaped mass occupying the anterior and posterior aspects of the right atrioventricular sulcus, measuring approximately 4×3 cm, which was hard in consistency [Table/Fig-1a]. The left atrioventricular sulcus also seemed to be involved macroscopically. With a provisional diagnosis of paediatric cardiac tumour, the dissection was undertaken. On opening the heart, the cardiac chambers were unremarkable, with normal wall thickness of all four chambers and interventricular septum. The mass was approached from the right atrium. The cut surface of the mass was reddish brown [Table/Fig-1b]. The right coronary artery was identified parallel to the nodular mass and its continuity was traced. The mass was not communicating with any of the heart chambers, great vessels or coronaries macroscopically. The cardiac valves, portions of the ascending aorta and pulmonary artery were of normal calibre and no thrombi were noted. Appropriate samples from cardiac mass, coronaries, great vessels, valves and cardiac chambers were taken. All other organs showed no significant macroscopic findings and were sampled for microscopic examination.
a) Gross photograph of the heart with a nodular mass (arrow) occupying the right atrioventricular sulcus; b) Cut section of the nodular mass occupying the anterior and posterior aspects of the right atrioventricular sulci. (View from right lateral aspect of heart).
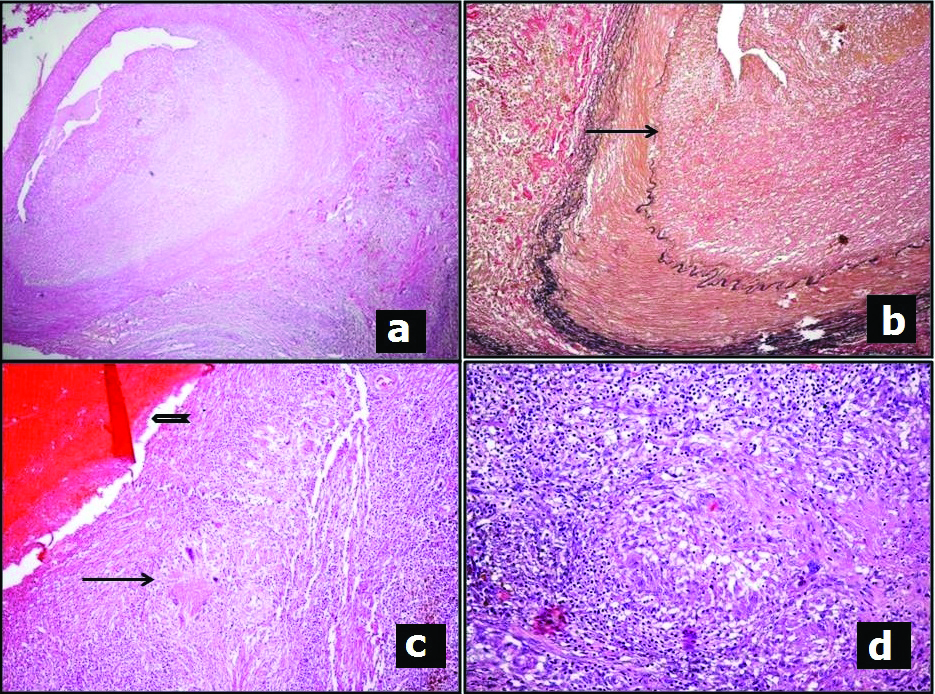
Microscopic examination of the sections from cardiac mass and coronary artery revealed remnants of a damaged vessel (the right coronary artery) in the pericardial fat. The arterial wall showed fibrosing granulomatous inflammation. The inflammation composed of neutrophils, lymphocytes, plasma cells and pigment laden macrophages [Table/Fig-2a,d]. The Elastic Van Gieson (EVG) stain revealed destruction and fragmentation of the internal elastic lamina with fibrosis [Table/Fig-2b]. The organising thrombus with calcification within the vessel wall and the inflammatory process resulted in dissipation of blood into the atrioventricular sulcus resulting in formation of the mass lesion seen adjacent to the artery, which indicated the chronicity of the lesion [Table/Fig-2c]. Further the right coronary artery was sampled at defined intervals from its origin. The arteritis was limited to the proximal 2.5 cm of the vessel and the distal segment was uninvolved. Sections from the root of aorta revealed similar inflammation with destruction of elastic fibres and fibrosis. The Ziehl Neilson stain for acid fast bacilli and fungal stains were negative. Microscopic examination of the other organs had no significant findings. Hence a possible diagnosis of Takayasu’s arteritis involving root of aorta and proximal segment of right coronary artery with secondary thrombus formation within the right coronary artery was rendered.
a) Sections from mass in right atrioventricular sulcus showing inflammation disrupting the wall of the right coronary artery (H&E,X40 original magnification); b) Elastic Van Geison stain highlighting disrupted (arrow) internal elastic lamina (X100 original magnification); c) Thrombus (open arrow) with adjacent granulomatous inflammation (arrow) (H&E,X40 original magnification); d) necrotising granulomatous inflammation in the epicardial tissue surrounding the right coronary artery (H&E X200, original magnification).
Discussion
Diseases of the heart account for 45-50% of all sudden deaths with Coronary Artery Disease (CAD) being the commonest cause. Sudden cardiac death in the young is most often the result of hypertrophic cardiomyopathy and coronary anomalies besides arrythmogenic causes (arrythmogenic right ventricular cardiac death dysplasia and long QT syndrome). Vasculitis is a rare cause of sudden death in children [1]. Vasculitis (inflammation of the blood vessels), is defined by the distribution of vascular and organ involvement that may involve only certain part of the arterial tree, veins or both. Coronary vasculitis may be encountered in association with various conditions like infections including tuberculosis, Kawasaki’s disease, metabolic disorders, metastatic disease and substance abuse [2]. It can be classified as either infectious (syphilitic, mycobacterial etc.) or autoimmune. Autoimmune coronary vasculitis is usually seen in the context of systemic autoimmune vasculitis and is rare in children, to result in sudden death [3].
Takayasu’s Arteritis, also known as aortoarteritis and pulseless disease, is a rare idiopathic condition. Takayasu’s arteritis is more common in young women particularly in Asians. The mean age at presentation of this disease in the Indian subcontinent is in the late second decade. In a study on 88 Indian patients, the mean age at symptom onset and diagnosis was 24 years and 28.3 years respectively [4]. It is a form of granulomatous arteritis, which affects large and medium sized arteries, primarily the aorta and its large branches as well as proximal portions of pulmonary, coronary and renal arteries [5]. It can involve the coronaries in 9 to 10% of the cases [6]. Multiple lesions involving coronaries in isolation or associated with aortic involvement are reported in the literature. However, vasculitis leading to sudden cardiac death in children is rare [7].
In the pathogenesis of this condition, the initial event is mononuclear cell infiltration in the adventitia of the large vessels with granulomas in the tunica media. This is followed by disruption of the internal elastic lamina and subsequent massive medial and intimal fibrosis. The vessel wall disruption followed by fibrosis results in segmental stenosis, occlusion, dilatation and aneurysmal formation in the affected vessels [8]. Association of HLA alleles and familial Takayasu’s arteritis are Increasing, being reported more commonly in Asian population especially from Japan [9].
The clinical presentation is highly variable and may present with constitutional symptoms in the initial phase I like low grade fever, malaise, night sweats, arthralgia, anorexia etc. As the disease progresses to phase II, tenderness over the vessels is seen followed by symptoms related to arterial stenosis or occlusion in phase III. Diagnosis of Takayasu’s arteritis relies on clinical presentation, structural arterial abnormalities and evidence of inflammatory vasculopathy either on imaging or histopathology [10]. A differential diagnosis of Takayasu’s arteritis should be considered in cases of pyrexia of unknown origin. Monitoring of disease activity is currently done with the help of clinical features combined with acute phase reactants like erythrocyte sedimentation rate and/or C reactive protein, imaging techniques like PET-CT, contrast enhanced MR, CT angiography and formal digital substraction arteriography. Pentraxin-3, produced by endothelial cells in response to inflammatory signals is believed to be an early marker of inflammatory activity in the vessels [10].
The EULAR/PRINTO/PRES criteria for diagnosis of Takayasu’s arteritis include aneurysm/dilatation of the vessels (mandatory criterion) plus one of the five minor criteria which include: pulse deficit or claudication, four limbs blood pressure discrepancy, bruits, hypertension, elevated acute phase reactant [11]. These criteria are entirely based on clinical and radiological findings making a retrospective diagnosis of Takayasu’s arteritis difficult in cases of sudden death in children. The disease can be asymptomatic as in the present case. A detailed post mortem examination with exclusion of involvement of other organs and documentation of active inflammatory phase is required to establish the diagnosis of Takayasu’s arteritis on autopsy [12].
Though the aetiology of Takayasu’s arteritis has not been completely elucidated, three possible mechanisms suggested include relationship to tuberculosis, genetic influences and immunologic mechanisms. Mycobacterial organisms has not been found in arteritic lesions, hence hypersensitivity to the the organism may be a possible pathogenic mechanism. Geographic distribution suggests genetic factors to be involved in the pathogenesis. Association with other autoimmune and collagen vascular diseases are also being investigated [13].
Besides Takayasu’s arteritis, other vasculitis leading to sudden death include eosinophilic (either associated or not with Churg-Strauss syndrome) vasculitis, lymphoplasmacytic vasculitis, idiopathic giant cell arteritis, vasculitis associated with systemic lupus erythematosus, Kawasaki disease and Polyarteritis nodosa [7]. The possibility of paediatric cardiac tumour was considered on macroscopic examination due to the mass formation seen involving the right and left atrioventricular sulci macroscopically. But on sectioning, it was seen as a haemorrhagic mass formation which was later confirmed on the representative histopathological sections. No aneurysmal dilatations were seen. Coronary artery vasculitis can present as intraluminal thrombosis as one of the complications besides aneurysm, stenosis and microcirculation abnormalities [14].
On histopathology, Takayasu’s arteritis is defined as large vessel vasculitis with inflammation involving all the three layers of the artery resulting in fibrosis leading to aneurysmal dilatations, stenotic lesions and thrombus formation. The inflammatory features vary depending on the phase of the disease-active inflammatory phase and chronic fibrosing phase, both can also co-exist [8]. The present case had areas of both active inflammation and fibrosis with superimposed complication of thrombus formation. Giant cell arteritis shows similar histopathological findings but tends to involve the cranial arteries in patients of older age group [15].
Conclusion
Vasculitis causing sudden cardiac death is uncommon in children, where coronary atherosclerotic disease is still the commonest cause. This report emphasises the need for detailed histopathological examination of the heart and its vessels in such cases to establish the nature of the disease and to ascertain the cause of death in a clinically asymptomatic and unsuspected case. With increasing evidence for familial occurrence of Takayasu’s arteritis, accurate diagnosis may warrant screening of the siblings and other family members.
Ethical approval: The consent for autopsy was obtained. The diagnosis was not known at the time of autopsy to obtain consent for publication. However, the report has no clinical photographs that reveal the identity of the patient. The patient’s family could not be contacted to obtain consent while the report was being written for publication.
[1]. Berger S, Utech L, Fran Hazinski M, Sudden death in children and adolescentsPaediatric Clinics of North America 2004 51(6):1653-77.10.1016/j.pcl.2004.07.00415561179 [Google Scholar] [CrossRef] [PubMed]
[2]. Mitchell RN, Schoen FJ, Robbins and Cotran Pathologic Basis of Disease 2014 8th edElsevier Health Sciences [Google Scholar]
[3]. Kedar R, Eisen A, Dovrish Z, Hadari R, Lew S, Amital H, Isolated coronary vasculitis as a cause of unexpected sudden deathIsr Med Assoc J 2009 11(12):769-70. [Google Scholar]
[4]. Subramanyan R, Joy J, Balakrishnan K, Natural history of aortoarteritis (Takayasu’s disease)Circulation 1989 80(3):429-37.10.1161/01.CIR.80.3.4292569946 [Google Scholar] [CrossRef] [PubMed]
[5]. Tan C, Qin W, Zhao Y, Yang Y, Huang Z, Liu Y, A case of isolated aorta occlusion caused by takayasu arteritisJournal of Clinical Rheumatology 2013 19(4):209-10.10.1097/RHU.0b013e318289dd0023669795 [Google Scholar] [CrossRef] [PubMed]
[6]. Matsubara O, Kuwata T, Nemoto T, Kasuga T, Numano F, Coronary artery lesions in Takayasu arteritis: Pathological considerationsHeart and Vessels 1992 7(S1):26-31.10.1007/BF01744540 [Google Scholar] [CrossRef]
[7]. Dermengiu D, Hostiuc S, Cristian Curca G, Constantin Rusu M, Paparau C, Ceausu M, Sudden death due to isolated segmentary coronary vasculitisThe American Journal of Forensic Medicine and Pathology 2014 35(4):223-31.10.1097/PAF.000000000000012525361059 [Google Scholar] [CrossRef] [PubMed]
[8]. Rizzi R, Bruno S, Stellacci C, Dammacco R, Takayasu’s arteritis: a cell-mediated large-vessel vasculitisInternational Journal of Clinical & Laboratory Research 1999 29(1):8-13.10.1007/s005990050055 [Google Scholar] [CrossRef]
[9]. Morishita K, Rosendahl K, Brogan P, Familial Takayasu arteritis - A Paediatric case and a review of the literaturePaediatric Rheumatology 2011 9(1):610.1186/1546-0096-9-621288360 [Google Scholar] [CrossRef] [PubMed]
[10]. Watson L, Brogan P, Peart I, Landes C, Barnes N, Cleary G, Diagnosis and assessment of disease activity in Takayasu arteritis: A childhood case illustrating the challengeCase Reports in Rheumatology 2014 2014:60317110.1155/2014/60317124511407 [Google Scholar] [CrossRef] [PubMed]
[11]. Ozen S, Pistorio A, Iusan S, Bakkaloglu A, Herlin T, Brik R, EULAR/PRINTO/PRES criteria for Henoch-Schonlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008. Part II: Final classification criteriaAnnals of the Rheumatic Diseases 2010 69(5):798-806.10.1136/ard.2009.11665720413568 [Google Scholar] [CrossRef] [PubMed]
[12]. Hlavaty L, Diaz F, Sung L, Takayasu arteritis of the coronary arteries presenting as sudden death in a white teenagerThe American Journal of Forensic Medicine and Pathology 2015 36(3):221-23.10.1097/PAF.000000000000017926110486 [Google Scholar] [CrossRef] [PubMed]
[13]. Gravanis M, Giant cell arteritis and Takayasu aortitis: morphologic, pathogenetic and etiologic factorsInternational Journal of Cardiology 2000 75:S21-S33.10.1016/S0167-5273(00)00184-4 [Google Scholar] [CrossRef]
[14]. Jeon CH, Kim YK, Chun EJ, Kim JA, Yong HS, Doo KW, Coronary artery vasculitis: Assessment with multidetector computed tomographyInternational Journal of Cardiovascular Imaging 2015 31(Suppl 1):59-67.10.1007/s10554-015-0652-825841665 [Google Scholar] [CrossRef] [PubMed]
[15]. Suresh E, Diagnostic approach to patients with suspected vasculitisPostgraduate Medical Journal 2006 82(970):483-88.10.1136/pgmj.2005.04264816891436 [Google Scholar] [CrossRef] [PubMed]