Lead is ubiquitous, versatile metal that has been used since pre historic times due to its excellent properties such as it is soft, silvery grey metal, melting at 327.5°C, highly resistant to corrosion, pliable, having high density, low elasticity, high thermal expansion, low melting point, easy workability, easily recycled, excellent antifriction and inexpensive. It has become extensively disseminated and mobilised in the environment; therefore, the human exposure and uptake of this element have consequently increased since last 20 decades [1]. Increased blood lead level can affect many organ systems, including the kidney, liver, gut, myocardium, the immune system, the peripheral and central nervous and at low blood lead level it affects the heme synthesis and other biochemical processes [2-4]. The first lead poisoning linked with occupational exposure was reported in 370 BC [5]. In 19th and 20th centuries, it become more common when workers were exposed to lead via various industrial activities such as smelting, painting, plumbing and printing. Lead exposure in these workers was mostly through the respiratory and Gastrointestinal (GI) Tracts. Approximately 30-40% of inhaled lead is absorbed into the blood stream [6]. In adult, the blood lead level below 10 μg/dL is normal; however in case of children even below 10 μg/dL is not safe. At present blood lead levels are considered as the most reliable index of lead exposure. Mainly >95% of blood lead is present in the erythrocytes and seems to be in dynamic equilibrium with plasma lead [7]. During increased absorption, it disseminated to the liver and kidneys and then accumulate in the bones and cause damage to mostly all the organs including liver, kidneys [8].
The silver jewellery manufacturing is mainly carried out at Hupari, Western Maharashtra (Dist. Kolhapur, India) and Silver Jewellery Workers (SJW) are smelting old silver ornaments and manufacturing the new silver jewellery at high temperatures in congested place without adequate exhaust system and such works are conducted in home. In silver jewellery industry lead is mainly used for manufacturing and designing of the silver rings. The workers involved in the production barely use any proper protective gears, such as mask or special aprons. In addition the family members are exposed to lead, since these manufacturing is mainly carried in small place of their houses. SJW are directly exposed to lead oxide dust and lead fumes, which results to increase lead absorption by ingestion, inhalation and direct skin contact and affects all organs and systems. In order to comprehend the present scenario, the present study was designed to measure the lead level in blood and its effects on liver and kidney function of occupationally lead-exposed SJW.
Materials and Methods
In this obeservational study 42 SJW and 50 normal healthy subjects were enrolled from Western Maharashtra, India (95 % Confidence Interval (CI) and 90% power of study). Subjects taking drugs for major illnesses were excluded from the study. Age range of the subjects under study was 20 to 60 years and age-matched control subjects were selected from the same area. Before the biological specimen collection, written consent was obtained from both the groups.
This research study was carried out at Krishna Institute of Medical Sciences “Deemed to Be University” Karad, Maharashtra, India. The research protocol was accepted by the institutional ethical committee (Ref. No. KIMSDU/IEC/03/2016, Dated 8/11/2016, Protocol No. 2016-2017/07) and maximum care was taken throughout the experimental procedure as per 1964 Helsinki declaration [9]. This study was conducted in April to June 2018. Blood samples from study and control group subjects were collected into CBC tubes.
Blood lead level was estimated using lead Care II blood lead analyser (Magellan Diagnostics Company, USA). The lead care II system is based on an electrochemical technique called Anodic Stripping Voltammetry (ASV) to determine the amount of lead in a blood sample. The blood was mixed with lead care treatment reagent (0.34 M-dilute hydrochloric acid solution in water), which lyses the red blood cells and release the lead. A negative potential was applied to the sensor to accumulate lead atoms on the test electrode. The potential is rapidly reversed releasing the lead ions. The current produced was directly proportional to the amount of lead in the sample [10].
Liver and kidney function tests were conducted using fully automated biochemistry analyser-EM360 Transasia. Serum AST and ALT transaminases activity were estimated by the UV-kinetic method using M/S Accurex Biomedical reagents. The rate of decrease in absorbance at 340 nm was measured, at the time of NADH to NAD conversion in both transaminase reactions [11].
Serum total protein was measured by the Biuret method. In alkaline pH the serum protein was reacted with cupric ion to form a violet coloured complex. The violet colour compound intensity was measured at 546 nm and it is directly proportional to serum protein concentration [12]. Serum albumin concentration was measured by the BCG method [13]. In acidic medium at pH 4.2 the serum albumin binds with 3,3’,5,5’-tetra Bromocresolsulfonapthalein (BCG) and forms the blue-green coloured complex and the colour intensity measured at 600 nm. The serum globulins were calculated by subtracting albumin from serum total proteins and the albumin/globulins ratio was calculated.
Serum total bilirubin was estimated by diazo method. Bilirubin is coupled with diazotized sulfanilic acid in the presence of ethylene glycol and dimethylsulfoxide as solvents to produce pink colour adiazo dye. The intensity of pink colour was measured at the 570 nm wavelength and proportional to the concentration of the bilirubin [14].
Serum Alkaline Phosphatase (ALP) was measured by using a 2-Amino-2-Methyl-1-Propanol (AMP). At the alkaline pH the 4-nitrophenol has an intense yellow colour, which is measured at 415 nm and the intensity of yellow colour is directly proportional to the serum ALP activity [15].
Serum creatinine was estimated by Jaffes method. In alkaline medium serum creatinine reacts with picric acid to produce an orange colour, which is measured at 492 nm and the intensity of orange colour is directly proportional to the concentration of serum creatinine [16].
Serum uric acid was measured by Uricase-Peroxidase (POD) method. Uric acid is oxidised by uricase to allantoin with the generation of hydrogen peroxide (H2O2). In the presence of peroxidase, a mixture of dichlorophenol sulphonate and 4-aminoantipyrine is oxidised by H2O2 to form a quinoneimine dye, which was measured at the 520 nm wavelength and proportional to the serum uric acid concentration [17].
Blood urea was measured by Glutamate Dehydrogenase (GLDH) method. Urea is decomposed by urease to form ammonia and carbon dioxide. Ammonia reacts with 2-oxo-glutarate in presence of NADH and glutamate dehydrogenase to form NAD and L-glutamate. The rate of NAD formation was measured at 340 nm and directly proportional to blood urea [18].
Statistical Analysis
Statistical analysis was done to compare blood lead levels, liver and kidney function tests of healthy controls and silver jewellery workers was done by Student’s t-test using Instant GraphPad software 8.
Results
Blood lead, serum levels of alanine transaminase, aspartate transaminase, bilirubin, alkaline phosphatase, creatinine, uric acid and blood urea were significantly increased while serum total protein, albumin and albumin/globulin ratio were significantly decreased in silver jewellery workers as compared to healthy control subjects [Table/Fig-1,2].
Blood lead levels and liver and kidney function tests of silver jewellery workers and normal healthy subjects.
| Biochemical Parameters | Control Group (N=50) | Silver Jewellery Workers (N=42) |
|---|
| A | Blood Lead (μg/dL) | 5.46±2.58 (3.3-11.9) | 23.23±5.91*** (11.9-37) |
| B. Liver Function Tests |
| 1. | AST (U/L) | 27.5±8.04 (16-49) | 32.23±12.68* (17-98) |
| 2. | ALT (U/L) | 28.87±9.1 (10-44) | 41.68±22.4** (19-108) |
| 3. | Total Proteins (gm/dL) | 8.08±0.67 (6.7-9.3) | 7.6±0.53*** (6.8-9.1) |
| 4. | Albumin (gm/dL) | 4.62±0.38 (3.8-5.2) | 4.07±0.17*** (3.8-4.5) |
| 5. | Globulin (gm/dL) | 3.5±0.7 (1.9-4.8) | 3.55±0.44# (2.9-4.6) |
| 6. | Albumin/Globulin Ratio | 1.39±0.45 (0.84-2.58) | 1.15±0.13** (0.89-1.35) |
| 7. | Bilirubin (mg/dL) | 0.53±0.2 (0.2-1.0) | 0.65±0.31* (0.4-1.8) |
| 8. | ALP (KA Units) | 63.4±21.08 (32-113) | 82.8±20.2*** (39-116) |
| C. Kidney Function Tests |
| 1. | Urea (mg/dL) | 20.5±4.78 (15-35) | 22.9±5.93* (15.8-36) |
| 2. | Uric acid (mg/dL) | 5.41±1.03 (3.1-8) | 6.39±1.18*** (4.3-9.9) |
| 3. | Creatinine (mg/dL) | 0.98±0.17 (0.3-1.3) | 1.12±0.17** (0.9-1.6) |
AST: Aspartate transaminase; ALT: Alanine transaminase; ALP: Alkaline phosphates
Figures indicate Mean±SD values and those in parenthesis are the minimum to maximum range of values of the specific group; *p<0.05, **p<0.01, ***p<0.001, #Non-significant as compared with controls (Student’s t-test)
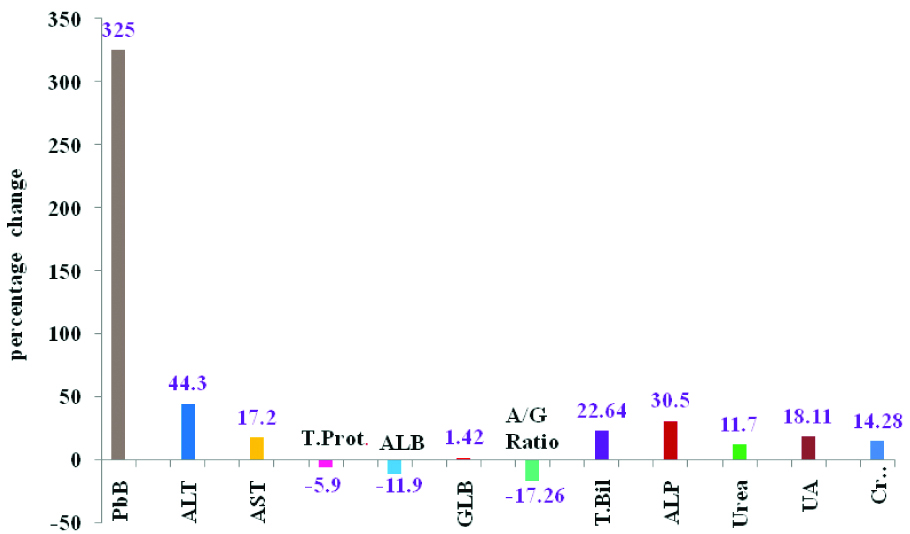
Percentage change of blood lead levels, liver and kidney function tests of Silver Jewellery Workers (SJW) with respect to healthy control subjects.
PbB: Blood lead; ALT: Alanine transaminase; AST: Aspartate transaminase; TP: Total proteins; Alb: Albumin; Glb: Globulin; A/G: Albumin/Globulin ratio; TBil: Total bilirubin; ALP: Alkaline phosphatase; BUL: Blood urea level; Creat: Creatinine; UA: Uric acid
Discussion
Bloodlead level was found to be significantly increased (p<0.001, 325%) in SJW as compared to healthy control subjects, indicating increased absorption of lead in SJW. In sliver jewellery industry, the workers are involved in various works such as smelting and alloying, rolling and milling, die-cutting and designing, assembling, soldering, polishing, plating of jewellery, and purification. In all these process the lead fumes and dust are generated and all these workers are exposed to lead. Lead wire is mainly used for manufacturing and designing the silver rings and workers involved in this business are highly exposed to lead [19]. The majority of silver jewellery workers have reported the clinical symptoms like reduced appetite, irregular abdominal pain, diarrhoea, muscle pain, nausea and constipation. All these clinical symptoms may be due to increased blood lead level.
The scanty ventilation, poor hygiene and lack of suitable protection might be the primary reason for increased blood lead level in SJW. The significantly increased blood lead level in SJW as compared to healthy control subjects have been reported by Goswami K et al., in 1999 and Patil AJ et al., in 2007 in previous studies [19,20].
Alanine transaminase (p<0.01, 44.3%), aspartate transaminase (p<0.05, 17.2%) and serum alkaline phosphatase (p<0.001, 30.59%) levels were also significantly increased in study group as compared to healthy control subjects [Table/Fig-1,2], indicates the slight alteration of liver functions. The increased levels of transaminases suggest necrosis of the hepatic cell by lead and lead induced hepatocellular injury. Mazumdar I et al., has observed increased liver enzymes level than controls in the chronic lead exposed workers [21]. Increased alkaline phosphatase levels may be an indicator of space occupying lesions in the liver.
Slightly decreased serum total proteins (p<0.01,-5.9%), albumin (p<0.01, -11.9%) and albumin/globulin ratio (-17.26%) was observed in SJW as compared to healthy control [Table/Fig-1,2]. The decreased level of serum total proteins and albumin might be due to the increased blood lead level in SJW. The increased blood lead level decreases the synthesis of plasma proteins mainly albumin reported in several earlier studies [20,22]. Therefore, the quantification of total protein was very helpful to identify the impairment of liver functions in lead exposure workers.
Serum total bilirubin level (p<0.01, 22.64%) significantly increased in SJW as compared to the healthy control subjects [Table/Fig-1,2], might be due to the increased blood lead level. Serum bilirubin concentration was significantly increased in lead exposed populations which were reported in earlier studies [23-28]. Serum bilirubin levels ranging from 1.5 to 2.5 mg/dL were common in lead poisoning cases. Lead causes hemolysis and resulting hemolytic jaundice and increased serum unconjugated bilirubin is well documented in the literature [23-28]. The administrations of large concentration of lead, which create morphological changes and destruction of red cells and increased the rate of breakdown of RBC, were reported in previous studies [30]. From the earlier studies [20, 29] and the present study it can be speculated that the slight increased serum bilirubin level in SJW as compared to healthy control group might be due to increased blood lead level.
The blood urea level (p<0.001, 11.7%), serum creatinine (p<0.001, 14.28%) and serum uric acid (18.11%) were significantly increased in study group as compared to the healthy control group [Table/Fig-1,2], might be due to high blood lead level and these results are also consistent with earlier reports in the literature [30,31]. The increased re-absorption of uric acid from the renal tubular cells resulting gout is a metabolic complication of lead induced renal impairment [32]. The higher levels of lead exposure results the impairment of renal functions reported in earlier study [33].
The results indicate that there was no additional precautions taken to reduce the lead exposure. Alterations of liver and kidney function test are not severe in SJW, however significantly increased blood lead levels were observed. Therefore, there is an urgent need to guard these workers from the health effects of occupational lead exposure by giving them the training for proper use and application of personal protective devices. Awareness should be created among all the workers and their family members regarding the occupational health hazards of lead, on how to reduce the lead exposure. The families of these workers residing in the same vicinity and hazardous conditions should also be screened for the blood lead levels. Moreover, treatment should be prescribed to those with increased blood lead level (>50 μg/dL).
Limitation
This study was conducted in small population of SJW and there is need to be known about the blood lead levels and its effects on liver and kidney function tests of the all silver jewellery workers from Hupari (Kolhapur).
Conclusion
Increased blood lead level in SJW of Western Maharashtra (India), indicating the higher absorption of lead, which slightly alters the liver and kidney function tests. The regular monitoring of blood lead level, liver and kidney function tests may prevent severe health hazards caused by lead.
AST: Aspartate transaminase; ALT: Alanine transaminase; ALP: Alkaline phosphates
Figures indicate Mean±SD values and those in parenthesis are the minimum to maximum range of values of the specific group; *p<0.05, **p<0.01, ***p<0.001, #Non-significant as compared with controls (Student’s t-test)