Pain is an unpleasant sensation localised to a part of the body and can be debilitating. Pain has a major impact on quality of life, sleep and socio-economic factors, such as one’s ability to adequately perform at work. Many chronic pain sufferers wind up taking huge amounts of analgesic drugs. Analgesic drug products that are used widely are opioids, acetaminophen and the Non-Steroidal Anti-Inflammatory Agents (NSAIDs), all of which have serious, potential toxicities, even when used in therapeutic doses.
Experimental pain models in healthy volunteers are advantageous for evaluation of analgesic action, as this is often difficult to assess in the clinic because of confounding factors such as sedation, nausea and general malaise, which may be present in a diseased condition. These pain models minimise the gap between knowledge gained in animal and human clinical studies. These tests measure subjective pain after pain stimuli [11].
Materials and Methods
The present study was conducted in the Department of Clinical Pharmacology and Therapeutics, NIMS, Hyderabad, over a period of six months from February 2011 to August 2011. The protocol was approved by the institutional ethics committee. (letter. No. IRB/NIMS/020/2010, issued on 18/11/2010). This was a randomised, double-blind, placebo-controlled, cross over study.
The current study was conducted as a part of thesis. So this was piloted at 70% power and 10% level of significance. To produce at least one second improvement in pain threshold (i.e., increasing the pain threshold by one second) compared to placebo, we needed 12 participants to complete this cross-over study. We have assumed that the intrasubject variability for Withania somnifera to be 1%. The testing procedures for analgesic activity were done by Ugo Basile Analgesymeter.
Twelve healthy male participants aged 18-40 years with normal BMI (19.5-25.9 kg/m2) and laboratory parameters were randomised to receive either placebo or Withania somnifera capsules. Participants who have consumed the analgesics (topical or systemic), history of allergy to study drugs, recent history of gastritis or gastric ulcer, history of finger deformities or fractures of tested hand were excluded from the study.
The study medication consisted of two capsules (500 mg each) of Withania somnifera and two placebo capsules, both given as single dose in a randomised manner, generated by the computer generated randomisation method. Placebo capsules were the identical look alike capsules of Withania somnifera, and they contained microcrystalline cellulose, lactose, magnesium stearate. Withania somnifera capsules were supplied by Natreon, Inc, USA contained a patented standardised aqueous extract of Withania somnifera. Each capsule contained an average of 15.7% of Withania glycosides, 40.2% of oligosaccharides and 0.24% of withaferin-A. Withania somnifera was selected for this study because withanolide glycosides (not the aglycones) are known to be the active ingredients, with oligosaccharides as bio-carriers and Withania somnifera contains very high levels of withanolide glycosides. The primary outcome measure of the study was to study the change in the pain threshold and tolerance force and time from baseline Withania somnifera. Safety and tolerability were studied as the secondary endpoints. Safety profile of Withania somnifera is well established and higher doses are well tolerated. Withania somnifera at 500 mg dose was not found to be an effective analgesic, 1000 mg dose was administered for this study.
The study was conducted in the healthy volunteers, wherein the volunteers underwent all the investigations to confirm their healthy condition like haemogram, Complete Urine Examination (CUE), renal function tests, hepatic function tests, electrocardiogram and random blood sugar. They were clinically examined by the physician. These healthy participants were briefed about the study during the initial consenting as well as post screening. They were explained and given demo on performing of the test (mechanical pain test, Randall Selitto test using Ugo Basile analgesymeter) on two different occasions prior to actual procedure.
Mechanical Pain Model (Randall Selitto Test using Ugo Basile Analgesymeter)
In the present study, we evaluated the analgesic activity of Withania somnifera using mechanical pain model by Randall Selitto test using analgesymeter (Ugo Basile analgesymeter) [18]. Clinical trial conditions were kept uniform for all the participants. They were healthy volunteers. On the day of the study, after good overnight sleep, participant was asked to report to the department at 7:00 AM in the morning in fasting state. They were given standard breakfast and were asked to sit in a quiet room with ambient temperature (22°C±2°C) and humidity for half an hour before the initiation of test procedure. Participant was asked to sit comfortably on a chair with his eyes blindfolded.
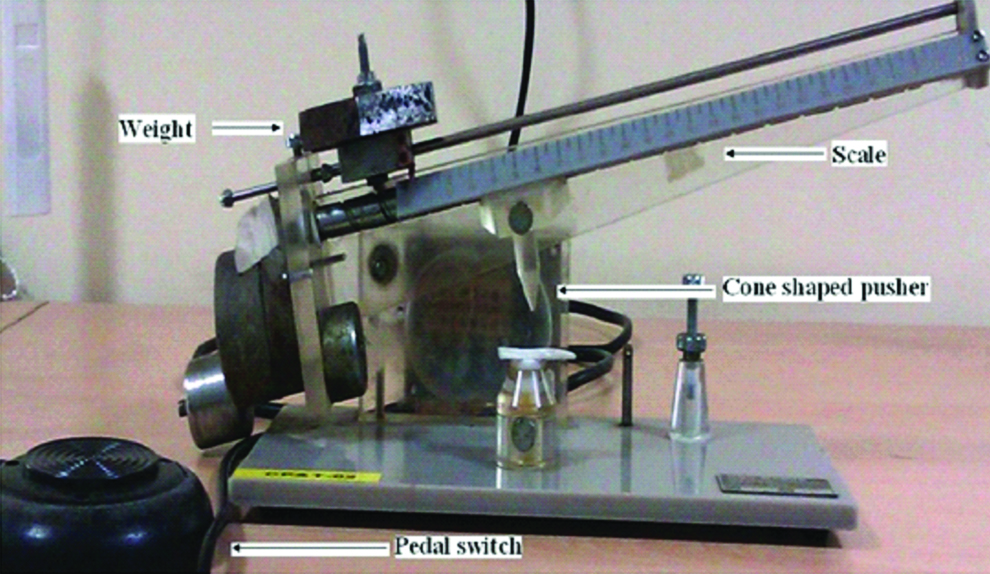
Initially participant’s index finger was placed on the instrument such that its nail bed is under the cone shaped pusher of the instrument [Table/Fig-1]. By pressing a pedal switch of the instrument, force was exerted by the pusher on the nailbed [Table/Fig-2]. The force was gradually increased at a constant rate of 80 grams per second. Readings on the instrument were noted when participant initially starts experiencing pain and again when he was unable to tolerate the pain. At that point (when he was unable to tolerate the pain), force on the nailbed is removed by simultaneous release of pressure on the pedal switch and lifting of the weight bearing arm of the instrument. The experiment was repeated three times at five minute intervals. The average of these readings was taken to calculate the pain threshold and tolerance force (in grams) and time.
Ugo basile analgesymeter.
Randall selittto test procedure.
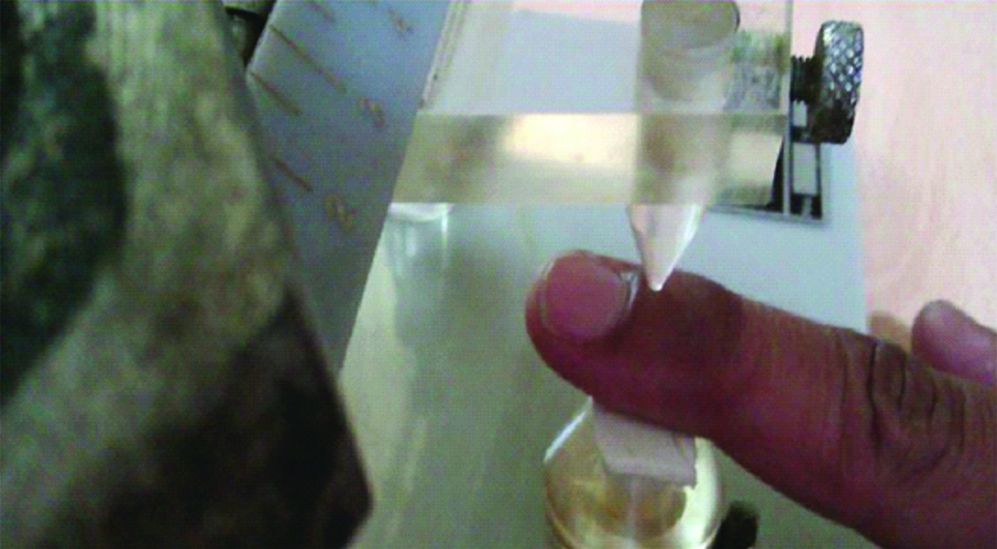
During the test procedure, when the subject first experiences pain (noted as pain threshold) and when he is unable to tolerate the pain (described as pain tolerance) the readings on the scale of the instrument were recorded and the force in grams and time in seconds were calculated.
The instrument has scale with readings on it. Instrument also has an arm on which weights can be added to it. For this study the blocks of weight are added to the arm such that each unit of the scale indicates 50 g of the force which is exerted on the nail bed. If the reading on the scale is 10, then the force exerted will be 500 g.
The volunteers were given either two capsules of 500 mg Withania somnifera extract or identical looking placebo capsules with 240 mL of water at 8:00 AM as per prior randomisation schedule, in a double blind manner. After taking the drug the subject was asked to sit upright on the chair for two hours. Randall Selitto test was repeated three hours after the drug administration and the readings were noted. After two weeks of wash out period, the second drug was administered according to the randomisation sequence and the same procedure was repeated. All safety laboratory parameters were repeated after administration of both drugs [Table/Fig-3].
The data on pain threshold and tolerance time were recorded in seconds and presented as mean±SD. The data on pain threshold and tolerance force were recorded in grams and presented as mean±SD. ANOVA and paired student t-test was used to compare the difference within the group at 80% power and p<0.05 was used to test the significance. All statistical analyses were performed using the Graph pad PRISM software 4 (Graph pad software Inc., USA).
Results
In this study, 14 male subjects were screened for participation into the study and of these there were two screen failures due to the presence of mild hypertension in both of them. The mean age was 34.25±3.112 years and average body mass index was 21.38±1.028 Kg/m2.
Evaluation of Pain Threshold: Mean pain threshold force with Withania somnifera increased from 450.29±75.49 to 538.5±72.27 grams (p=0.003). Mean threshold force for pain with placebo increased from 443.06±65.02 to 446.53±65.95 grams (p=0.44) [Table/Fig-4]. Pain threshold time with Withania somnifera was increased from 5.63 seconds to 6.73 seconds (p=0.003). Pain threshold time with placebo increased from 5.54 to 5.58 seconds (p=0.50) [Table/Fig-4].
Pain threshold force and time with Withania somnifera (WS) and placebo at baseline and post drug.
| Withania somnifera | Placebo |
|---|
| Threshold Force (grams) |
| Baseline | 450.29±75.49 | 443.06±65.02 |
| Post drug administration | 538.5±72.27*# | 446.53±65.95 |
| Threshold Time (seconds) |
| Baseline | 5.63±0.94 | 5.54±0.81 |
| Post drug administration | 6.73±0.90*# | 5.58±0.82 |
(All values Mean±Standard deviation)
*-P-0.003 compared to baseline, #-p-0.000052 compared to placebo
Evaluation of Pain Tolerance: Mean pain tolerance Force with Withania somnifera increased from 806.9±61.36 to 878.2±82.6 grams (p=0.002). Mean tolerance force for pain with placebo increased from 800.69±90 to 802.08±84.25 grams (p=0.514) [Table/Fig-5]. Mean pain tolerance time with Withania somnifera has increased from 10.09 seconds to 10.97 seconds (p=0.002). Pain tolerance time with placebo increased from 10.01 to 10.03 seconds (p=0.476) [Table/Fig-5].
Pain tolerance force (grams) and time (seconds) with Withania somnifera and placebo at baseline and post drug.
| Withania somnifera | Placebo |
|---|
| Tolerance Force (grams) |
| Baseline | 806.9±61.36 | 800.69±90.00 |
| Post drug administration | 878.2±82.60*# | 802.08±84.25 |
| Tolerance Time (seconds) |
| Baseline | 10.09±0.77 | 10.01±1.12 |
| Post drug administration | 10.97±1.03*# | 10.03±1.05 |
(All values Mean±Standard deviation)
*-p<0.002 compared to baseline, #-p-0.0000052 compared to placebo
When compared to baseline, there was 19.6% increase in pain threshold force and time post drug with Withania somnifera, while it was only 0.78% with placebo. Furthermore, when compared to baseline, pain tolerance force and time post drug increased by 8.83% with Withania somnifera, but with placebo it was only 0.17% [Table/Fig-6].
Percentage increase in the pain threshold and pain tolerance force and time before and after drug (Withania somnifera and Placebo) administration.
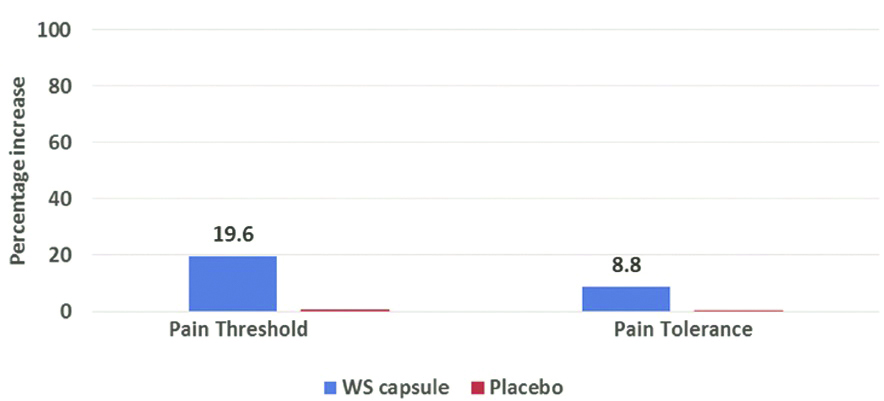
The difference in the improvement of pain threshold and pain tolerance force and time from baseline to post drug were statistically more for Withania somnifera when compared to that achieved with placebo (p<0.0000052).
Compliance was assured, as the drugs were administered under supervision. Both the drugs were well tolerated and none of the subjects reported any adverse events. All safety laboratory parameters were repeated after the drug administration and they were found to be normal.
Discussion
In the present study, Withania somnifera when compared to placebo significantly increased the pain threshold and pain tolerance force and time. Withania somnifera (Ashwagandha) is widely used in Ayurvedic medicine as a part of polyherbal formulations.
Withaniasomnifera when studied in rats also showed analgesic, anti-pyretic and anti-inflammatory properties without producing gastric ulceration [19]. A wide variety of Withania-derived chemicals such as withanolides (Withaferin A) were found to have remarkable antinociceptive activity in swiss albino mice [10]. In our study, aqueous extract of Withania containing preparation Withania somnifera has demonstrated analgesic activity in the experimentally induced mechanical pain in healthy human volunteers.
A poly herbal formulation containing Withaniasomnifera extracts was studied for the treatment of pain in osteoarthritis, wherein it was found that the poly herbal formulation significantly reduced the severity of pain (p<0.001) and disability (p<0.05) scores, although no significant changes in radiological appearance or Erythrocyte Sedimentation Rate (ESR) were noted [20]. Another poly herbal preparation (Ammukkara choornam) containing Withania somnifera was also evaluated in the rheumatoid arthritis patients objectively with respect to improvement of ESR, alkaline phosphatase, rheumatoid factor and concluded that objective improvement in ESR, alkaline phosphatase and rheumatoid factor may be well correlated to produce symptomatic relief in rheumatoid arthritis [14].
In the present study, we used Ugo Basile Analgesymeter for conducting Randal Selitto test for inducing mechanical pressure on the nail bed of non-dominant hand index finger, as it is associated with good precision and smaller interindividual and intraindividual variation of baseline effect [21,22]. This test is having good correlation with the results of the cold stimulation test [23], which is considered to be sensitive method for evaluation of drugs having central mechanism of action. Randall-Selitto test was used for evaluation of other herbal preparations having analgesic and anti-inflammatory properties like the extract from Stereospermum Kunthianum [24], wherein they studied the analgesic and anti-inflammatory activities of the compounds isolated from the bark of Stereospermum kunthianum and concluded that the compounds had significant anti-inflammatory and analgesic properties.
Randall and Selitto test is one of the most predictive of the models of acute pain the other being, the formalin test [25]. The other method to induce mechanical pressure is Sphygmomanometer cuff used by Ryan ED and Kovacic CR [26]. Besides inducing pressure pain, the sphygmomanometer also induces ischaemic pressure on the calf muscles. This might cause disturbance to the subject’s perception of the pressure pain, and thereby, decreasing the specificity of the pressure pain test.
In the present study, single dose Withania somnifera aqueous extract 1000 mg was well tolerated without producing any adverse effects. Similar results were reported in a dose ranging, tolerability study, of aqueous extract of Withania somnifera in healthy human volunteers and it was found that Withania somnifera up to 1250 mg was well tolerated [27]. Review of literature also showed that, Withania somnifera containing polyherbal preparations were not found to have any major adverse effects in patients suffering from osteoarthritis, rheumatoid arthritis [14,20].
Limitation
This was a healthy volunteer study and the mechanical pain was induced using mechanical pain model. Literature evidence shows that Withania somnifera has analgesic activity, so we have piloted this study at lower power and higher alpha. Analgesic activity of Withania capsules was evaluated by Randall Selitto test. As our results show the drug has shown analgesic activity, this would be further confirmed by studying in patient suffering with rheumatoid arthritis with higher power and significance level. In this study only male subjects were enrolled to avoid any hormonal effect causing variations in the response to drug, so this effect will be studied in the future studies by enrolling even the female patients.
Conclusion
In this present study, Withania somnifera when compared to placebo significantly increased the pain threshold and pain tolerance force and time. Both the drugs were well tolerated and no adverse events were observed during the study. Further studies are needed with Withania somnifera in patients suffering from osteoarthritis, rheumatoid arthritis and other pain conditions requiring analgesics. Withania somnifera may prove to be safe and effective alternative to patients with painful rheumatological disorders.
(All values Mean±Standard deviation)
*-P-0.003 compared to baseline, #-p-0.000052 compared to placebo
(All values Mean±Standard deviation)
*-p<0.002 compared to baseline, #-p-0.0000052 compared to placebo