Materials and Methods
Present experimental work was conducted at Srinivas Institute of Medical Sciences, Mukka, Mangaluru, and SDM Centre for Research in Ayurveda and Allied Sciences Udupi, for three years nine months (September 2012-June 2016). The institutional animal ethical committee approval was taken before the experiment with the reference number SDMCA/IAEC/CPCEA/GI04/2011.
Test Drugs and their Dose Level
The human dose concentration of copper formulated Tamra Bhasma is 22 mg/kg/body weight [9], whereas the dosage experimental animal was estimated by body surface area ratio by referring to the standard table of Paget & Barnes [10] and on this basis of conversion factor, animal dose level determined to be 1.98 mg/kg (therapeutic dose) and 9.9 mg/kg body weight (five times of therapeutic dose). Similarly, the human dose concentration of zinc formulated Jasada Bhasma is used for the therapeutic activity is 120 mg/kg/body weight. However, for the experimental purpose animal dose was estimated to be 10.8 mg/kg (therapeutic dose) and 54 mg/kg body weight (five times of therapeutic dose) [4].
Accordingly, working dose of both the test drug was suspended in 0.5% Carboxymethyl Cellulose (CMC) based upon the body weight of animals (1 mL/100 g body weight) was prepared separately and the same was administered orally by using a catheter.
Study Design
Total of 25 Wistar albino male rats weighing 150-225 g body weight were used divided into five different groups randomly. Each group comprised of five rats. All the animals were fed with rat diet and water, add libitum and acclimatised them to the standard laboratory conditions.
Group-I rats were used as the control and received only vehicle (0.5% CMC). Group-II rats received Tamra Bhasma at a therapeutic dose (TED) 1.98 mg/kg. Group-III rats received Tamra Bhasma at five times of therapeutic dose (TED×5), i.e., 9.9 mg/kg body weight. Group-IV rats received Jasada Bhasma at a Therapeutic Dose (TED) of 10.8 mg/kg body weight, and Group-V rats received Jasada Bhasma five times of Therapeutic Dose (TED×5), i.e., 54 mg/kg body weight.
Both the test drugs were administered to the specified groups for 28 consecutive days [11]. On the 28th day, all the animals from each group were sacrificed, and the testes of each animal were dissected out for the gross weight changes among the group animals and histopathological investigations [12,13]. The routine Haematoxylin and Eosin (H&E) stain has been employed on the paraffin sections, and the cytoarchitecture of testicular morphology has been studied under the light microscope.
Statistical Analysis
The data obtained were expressed in mean±SEM and statistical analysis of variance (ANOVA) test performed using SPSS version 16.0, for determining the level of significance of the observed effects. The p-value of less than 0.05 was considered to be statistically significant.
Results
The data related to the effect of Tamra Bhasma and Jasada Bhasma on the gross weight of testes has been depicted in the [Table/Fig-1]. The data showed that there is an apparent increase in testis weight in Tamra Bhasma and Jasada Bhasma treated groups compared to normal control at both TED and TED×5 dose levels. However, the values were statistically significant only at TEDx5 dose levels (p<0.001).
Effect of Tamra Bhasma and Jasada Bhasma on testis weight.
| Group | Testis weight (g) Mean±SEM | % Change |
|---|
| I. Control | 2.57±0.15 | - |
| II. Tamra Bhasma at TED | 2.72±0.09 | 8.81 ↑ |
| III. Tamra Bhasma at TEDX5 | 2.93±0.14 | 14.22 *↑ |
| IV. Jasada Bhasma at TED | 2.73±0.15 | 8.61 ↑ |
| V. Jasada Bhasma at TEDX5 | 2.94±0.06 | 14.42* ↑ |
TED-Therapeutic dose level, TED×5-five times of therapeutic dose level, ↑ increased change
Data expressed in Mean±SEM, *The mean difference is significant at 0.001 level in comparison to normal control
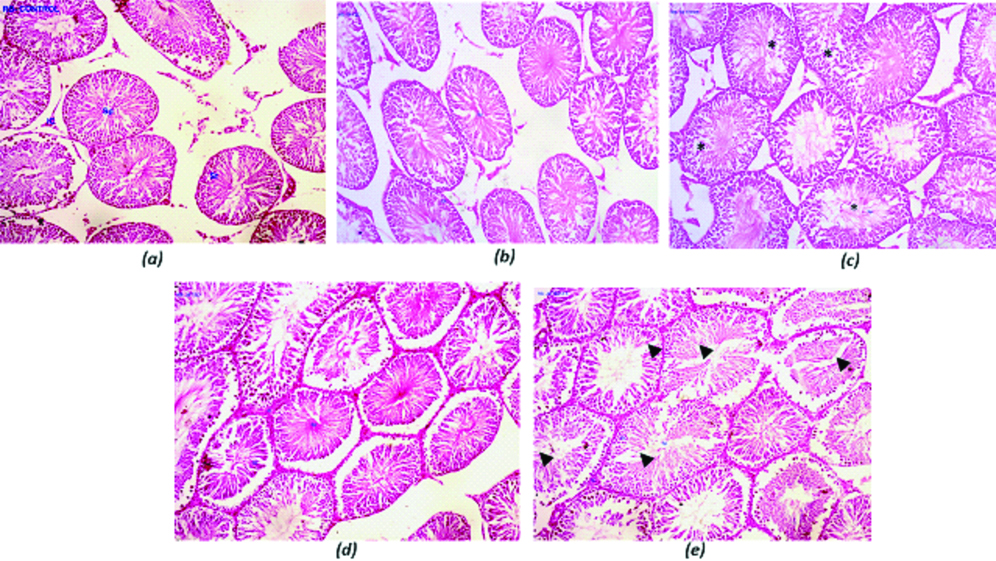
The histological sections of the testis of rats treated with Tamra Bhasma and Jasada Bhasma at the therapeutic dose level (i.e., group II and IV rats) did not show any toxic changes so that they retained their cytoarchitecture as normal as that of normal control (group I) rats [Table/Fig-2a,b,d]. However, slight morphological variation owing to altered spermatogenic features than that of normal features was observed in the testicular tissues of the rats group (III) treated with five times of therapeutic dose of TamraBhasma [Table/Fig-2c,3a]. But, remarkably decreased spermatogenic architecture at test drug administered at the therapeutic dose of Jasada Bhasma in group V rats were apparent histologically as there are many vacuoles in the various stages of spermatogenesis in more number of seminiferous tubules [Table/Fig-2e,3b].
Showing the histopathological features of testis when compared to normal: (a) to that of exposure to TamraBhasma at therapeutic dose; (b) and at five times of therapeutic dose levels; (c) JasadaBhasma at therapeutic dose; (d) and at five times of therapeutic dose; (e) levels. Asterisks (in c) and thick arrow heads (in e) are showing the degenerative changes in the seminiferous tubules.
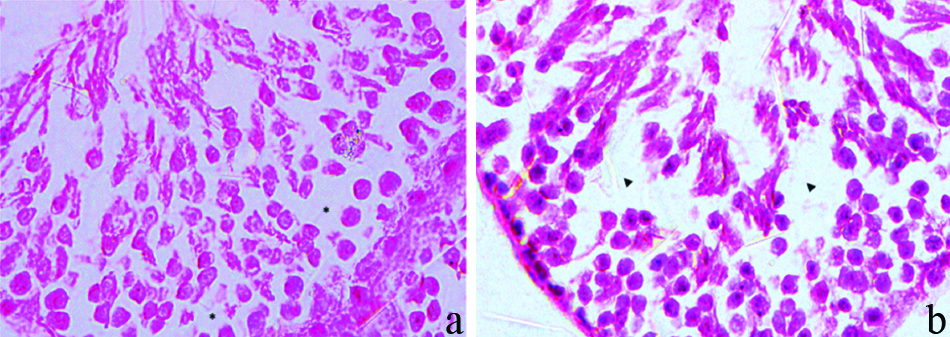
High magnification figures of testes sections exposed to five times of therapeutic dose of TamraBhasma (a) and JasadaBhasma (b) with the features of degenerative changes in the form of vacuoles within the stratified epithelium lining of the seminiferous tubules (H&E stain, 100x).
Discussion
Evaluation of safety and efficacy of mineral-based preparations need to be carefully monitored in the field of biomedical sciences. A novel approach in this regard by Bhaskar R et al., reported useful findings in the scientific literature [5,8]. The histomorphology of various vital organs studied at the exposure of both the dose levels (TED and TEDx5) revealed no remarkable toxicity changes except a mild sinusoidal dilation in the liver with moderate diffused micro fatty changes and necrotic changes in tubular epithelium of the kidneys at higher concentrate dose levels of both Bhasmas [5,8]. These minor histopathological changes in the organs were perhaps due to incomplete processing of the formulations of Bhasmas. Improper processing of the Bhasmas known to contaminate with the metal ion impurities which could result in various adverse effects and toxicity following its therapeutic usage in higher concentration [6].
In the present study, an attempt has been made to evaluate the efficacy and safety use of Tamra Bhasma and Jasada Bhasma on the male reproductive organ of the Wistar albino rats. Like other organs, the testis of the experimental rats treated with these Bhasmas at usual Therapeutic Dose Level (TED) was not showing any toxicity changes in the histological study. However, upon the exposure to a higher concentration level (TED×5), the histological features were suggestive of decreased spermatogenesis and moderate oedematous changes. These features were more apparent in Jasada Bhasma exposed testis compared to that of Tamra Bhasma with the evident of observable morphological alteration in the cyto-architecture. In supportive of this, the significant increase in gross weight of the testes following the exposures to higher concentrations of both Bhasmas is a clear indication of its adverse effect.
Sanjay Kumar YR et al., reported safety usage of Tamra Bhasma upto dose 13.5 mg/kg body weight for 28 days and 90 days for sub-acute toxicity and chronic toxicity respectively, in Wistar rats [11].
Previous studies have also witnessed the effective and safe use of Jasada Bhasma provided it is properly processed and administered. It implies, the different chemical compositions and biological activities of the bhasma preparations said to have specific clinical efficacy depending on the media of its preparation [14].
Since the organ weight is a sensitive index in any pathological scenario, increased weight may be suggestive of oedema, hyperplasia or hypertrophy of the tissues. The present study observation confirms safer usage of both Tamra Bhasma and Jasada Bhasma at its therapeutic dose level, than that of its higher concentration as used in the current experimental study. At five-times therapeutic dosage, the weights of the testes have remarkably increased probably owing to mild oedematous changes that could be an indication for the decreased spermatogenesis.
Thus, the overall histopathological examinations exhibiting some changes in testes sections deserve some attention while administration of Bhasmas of higher concentration with testicular functional monitoring would be a prudent step in clinical settings. Though the usage of Tamra Bhasma is relatively safer than Jasada Bhasma, the metal Tamra is attributed with AshtaMahaDosha because of the toxic nature due to its improper purification [15]. Therefore, one should be cautious while using Tamra Bhasma. Meanwhile, the possible immune-modulatory quality of Jasada Bhasma to have protective role against systemic level damage by lowering the oxidative stress in the organs as quoted by Chandran S et al., is also cannot be ignored [14].
Limitation
The prolonged duration of exposure of higher dose level may completely arrest the spermatogenesis. So, extended follow-up should have been carried out which is the limitation of the present study. In future, comprehensive purification in the preparation of Bhasmas using analytical and imaging techniques is warranted to determine its particle size, density together with its physical and chemical stability. It is essential to rule out the effect of these test drugs causing changes at the molecular level to correlate with the significant mutagenesis potential. So genotoxicity profile of these formulations could give more relevant results in supportive of our findings.
Conclusion
In the present study, we have evaluated a toxicity test on the testes by the usage of two popular Bhasma formulations popularly used in Indian system of medicine. From the acute and sub-acute toxicity study, we can conclude that test drugs were relatively safer and did not produce any marked toxic effects at their therapeutic dose levels. However, at higher doses both drugs may produce toxicity on male gonads, which could have an adverse effect on spermatogenesis. The higher dose used in the present study was much higher than routinely practiced clinical doses. Therefore, dose-dependent usage of these Bhasmas is recommended.
Scope for Further Study
It is essential to rule out the effect of these test drugs causing changes at the molecular level to correlate with the significant mutagenesis potential. So, genotoxicity profile of these formulations could give more relevant results in supportive of our findings.
TED-Therapeutic dose level, TED×5-five times of therapeutic dose level, ↑ increased change
Data expressed in Mean±SEM, *The mean difference is significant at 0.001 level in comparison to normal control